MINNEAPOLIS — The rapid expansion of treatment options for advanced or metastatic renal cell carcinoma (RCC) in the last decade has improved survival rates and brought hope to the one-third of RCC patients diagnosed with Stage IV cancer. It also has increased the challenge physicians face in selecting the best therapy for each patient.
The first immunotherapy, the monoclonal antibody nivolumab, received Food and Drug Administration approval in 2015 for advanced renal cell carcinoma. Since then, multiple monoclonal antibodies, tyrosine kinase inhibitors and other immunotherapies have gained approval for RCC including avelumab, axitinib, bevacizumab, belzutifan, cabozantinib, ipilimumab, pembrolizumab and tivozanib.
While monotherapy still has a place in relapsed and advanced RCC, combination therapies have come to dominate first-line treatment. In the March 2024 National Comprehensive Cancer Network guidelines, all the preferred, Category 1 first-line regimens are combinations: axitinib with pembrolizumab, cabozantinib with nivolumab and lenvatinib with pembrolizumab for all risk groups and ipilumumab with nivolumab for those with poor or intermediate risk.
Among those options, though, which one is best? Philipp Dahm, MD, director of Research and Education for Surgical Services at the Minneapolis VAMC, professor of urology and vice chair of Veterans Affairs at the University of Minnesota and his colleagues at Cochrane sought to find out.
Their meta-analysis included 36 randomized controlled trials with a combined 15,177 participants and assessed overall survival, quality of life and serious adverse events. The combinations evaluated included pembrolizumab with axitinib (PEM+AXI), avelumab with axitinib (AVE+AXI), nivolumab plus cabozantinib (NIV+CAB), lenvatinib with pembrolizumab (LEN+PEM) and nivolumab with ipilimumab (NIV+IPI) as well as the monotherapies cabozantinib and pazopanib.
Because of a lack of head-to-head studies, the combinations were compared to sunitinib, an earlier-generation preferred therapy.
Overall Survival
Looking at overall survival across favorable, intermediate and poor risk groups, the researchers found that, compared with sunitinib, two combinations probably improve outcomes: pembrolizumab with axitinib (HR 0.73, 95% confidence interval (CI) 0.50 to 1.07, moderate certainty) and nivolumab plus cabozantinib (HR 0.69, 95% CI 0.69 to 1.00, moderate certainty). There was less certainty of the overall survival benefit with lenvatinib plus pembrolizumab (HR 0.66, 95% CI 0.42 to 1.03, low certainty) when compared to sunitinib. Of the two monotherapies, pazopanib demonstrated little benefit compared to sunitinib (HR 0.91, 95% CI 0.64 to 1.32, moderate certainty) and cabozantinib appeared to modestly improve overall survival compared to sunitinib, but the results were not statistically (HR 0.84, 95% CI 0.43 to 1.64, very low certainty).
The median survival is 28 months for patients treated with sunitinib. The researchers found that nivolumab plus cabozantinib and pembrolizumab with axitinib probably improve overall survival to 41 months and 39 months, respectively. With less certainty, lenvatinib plus pembrolizumab may push overall survival to 43 months, and pazopanib likely brings overall survival to 31 months with PAZ.
The team was uncertain whether survival improves to 34 months with cabozantinib. Comparison data were unavailable for avelumab with axitinib and nivolumab plus cabozantinib.
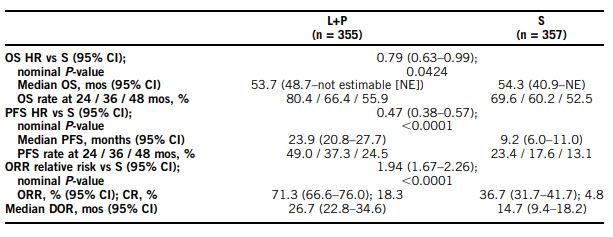
More recently, the final prespecified overall survival analysis of the phase III CLEAR trial presented at the 2023 ASCO Annual Meeting found that, at extended follow-up, lenvatinib plus pembrolizumab showed sustained superiority over sunitinib for overall and progression-free survival as first-line treatment for advanced renal cell carcinoma.The majority of the benefit was observed in intermediate and poor-risk subgroups.1
At a median follow-up (IQR) of 49.8 mos (41.4-53.1) for lenvatinib plus pembrolizumab and 49.4 mos (41.6-52.8) for sunitinib, 149 and 159 deaths had occurred, respectively. The researchers noted that overall survival benefit with the lenvatinib plus pemrolizumab combination was maintained (HR, 95% CI; 0.79, 0.63-0.99), and that overall survival favored lenvatinib plus pembrolizumab versus sunitinib across Memorial Sloan-Kettering Cancer Center (MSKCC/Motzer) Score for Metastatic Renal Cell Carcinoma (RCC) risk groups (HR, 95% CI; favorable [fav]: 0.89, 0.53-1.50; intermediate [int]: 0.81, 0.62-1.06; poor: 0.59, 0.31-1.12).
In addition, the progression-free survival benefit of PFS benefit of lenvatinib plus pembrolizumab versus sunitinib was maintained (HR, 95% CI; 0.47, 0.38-0.57), according to the authors.
The presenters concluded that lenvatinib plus pembrolizumab “continues to demonstrate clinically meaningful benefit vs S in OS, PFS, ORR, and CR in the 1L treatment of pts with aRCC at 4-yr follow-up, thus supporting the robustness of the primary analysis data from CLEAR.”
Quality of Life
In the VA lead review, only one trial measured quality of life. Using FACIT-F (score range 0 to 52; with higher scores indicating better quality of life), the study found that the mean post-score was 9.00 points higher (9.86 lower to 27.86 higher, very low certainty) with pazopanib than with sunitinib. Comparison data for the other combinations and cabozantinib were not available.
Serious Adverse Events
Across risk groups, pembrolizumab with axitinib probably increases the risk for serious adverse events the least, compared with sunitinib (RR 1.29, 95% CI 0.90-1.85, moderate certainty). In comparison, lenvatinib with pembrolizumab (RR 1.52, 95% CI 1.06-2.19, moderate certainty) and nivolumab with ipilimumab (RR 1.40, 95% CI 1.00-1.97, moderate certainty) probably increase the risk for SAEs somewhat more, compared to sunitinib, respectively. The risk appears to be the same for SAEs for patients taking pazopanib or sunitinib (RR 0.99, 95% CI 0.75-1.31, moderate certainty), while the researchers were uncertain about the impact on SAE risk for cabozantinib versus sunitinb (RR 0.92, 95% CI 0.60-1.43, very low certainty).
The mean risk of SAEs for patients taking sunitinib is 40%. The risk increases probably to 61% with lenvatinib with pembrolizumab, 57% with lenvatinib with pembrolizumab, and 52% with pembrolizumab with axitinib, while probably staying at 40% for pazopanib, according to the review. There was significant uncertainty about the risk of SAEs with cabozantinib and comparison data were not available for avelumab with axitinib and nivolumab plus cabozantinib.
- Aldin A, Besiroglu B, Adams A, Monsef I, et. Al. First-line therapy for adults with advanced renal cell carcinoma: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2023 May 4;5(5):CD013798.
- Motzer RJ, Porta C, Eto M, et al: Final prespecified overall survival analysis of CLEAR: 4-year follow-up of lenvatinib plus pembrolizumab vs sunitinib in patients with advanced renal cell carcinoma. 2023 ASCO Annual Meeting. Abstract 4502. Presented June 5, 2023.