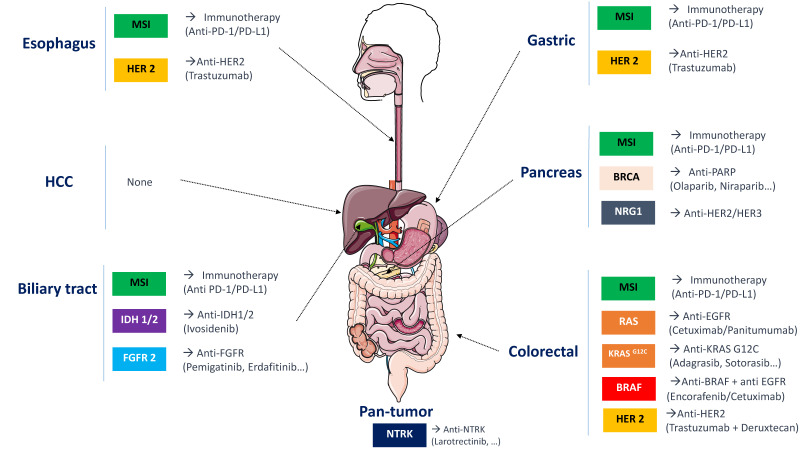
Click to Enlarge: Major molecular abnormalities in digestive cancers and current treatment options.
Source: MDPI: Cancers
WASHINGTON, DC — Recent research in metastatic colorectal cancer (mCRC) demonstrates that personalized therapy outperforms broad approaches to treatment of this challenging malignancy in federal medicine and elsewhere.
With a growing number of therapies available, selecting the right drug for each patient becomes ever more important, both for achieving the best outcomes and for minimizing adverse events caused by drugs unlikely to benefit the particular patient.
The concept of “negative hyperselection” has emerged as a pivotal strategy to optimize therapeutic outcomes, particularly concerning the use of anti-epidermal growth factor receptor (EGFR) therapies such as panitumumab. This approach involves the comprehensive exclusion of patients harboring genetic alterations associated with resistance to anti-EGFR treatments. By refining patient selection through detailed molecular profiling, clinicians can enhance the efficacy of targeted therapies and improve patient prognoses.
Traditionally, the selection of patients for anti-EGFR therapy in mCRC has been based on the absence of RAS mutations, as these mutations are well-established predictors of resistance. Emerging evidence, however, indicates that other genetic alterations, such as mutations in BRAF, PIK3CA, and PTEN, as well as HER2 amplifications, can also confer resistance to anti-EGFR agents. Negative hyperselection entails the exclusion of patients with any of these mutations and treating only RAS wild-type (RASwt) mCRC patients who are most likely to benefit from therapies like panitumumab.1
Clinical Implications of Negative Hyperselection
Implementing negative hyperselection in clinical practice necessitates comprehensive molecular profiling of mCRC tumors. Next-generation sequencing (NGS) technologies enable the detection of a broad spectrum of genetic alterations, facilitating the identification of patients who are most likely to respond to anti-EGFR therapies. This approach not only maximizes therapeutic efficacy but also spares patients unlikely to benefit from potential treatment-related toxicities.
Moreover, the dynamic nature of tumor genetics implies that resistance mutations can emerge over time, especially under therapeutic pressure.2 Regular monitoring through liquid biopsies, which analyze circulating tumor DNA (ctDNA), offers a non-invasive means to detect these alterations in real-time as they develop. This allows for timely adjustments in therapeutic strategies to ensure treatment plans remain aligned with the evolving molecular landscape of the tumor.
A study published in Nature Medicine provides compelling evidence supporting the utility of negative hyperselection in optimizing treatment outcomes for mCRC patients. This research emphasizes the prognostic significance of baseline ctDNA profiling in predicting patient responses to combined panitumumab and chemotherapy regimens.3
The phase III PARADIGM trial demonstrated longer overall survival with first-line panitumumab vs. the antivascular endothelial growth factor bevacizumab when both drugs were added to modified 5-fluorouracil, l-leucovorin, oxaliplatin (mFOLFOX6) chemotherapy in patients with RASwt mCRC with left-sided primary tumors.4
In the Nature Medicine study, researchers conducted an exploratory biomarker analysis within a 733-person subset of the PARADIGM participants to determine whether specific genetic alterations detected in baseline ctDNA samples could predict survival outcomes in this setting.
The study focused on gene alterations in KRAS, NRAS, PTEN and extracellular domain EGFR mutations, HER2 and MET amplifications as well as ALK, RET and NTRK1 fusions. Comparing outcomes from patients with any of these mutations to those of patients whose ctDNA lacked those specific alterations at baseline found the latter group experienced significantly improved overall survival compared to those with detectable mutations, suggesting the absence of these particular genetic alterations resulted in better response to panitumumab-based therapies.
For patients with left-sided primary tumors who met negative hyperselection criteria, panitumumab + mFOLFOX6 extended overall survival compared to bevacizumab + mFOLFOX6 in patients (median 42.1 versus 35.5 months; HR, 0.76; 95% CI, 0.61–0.95). In addition, the study found higher rates of antitumor response (83.3% versus 66.5%), curative resection (19.8% versus 10.6%) and greater depth of response (median, −60.2% versus −43.6%) with panitumumab versus bevacizumab.
“Our results suggest that negative hyperselection using a validated and adequately sensitive plasma ctDNA assay may inform appropriate selection of patients for panitumumab treatment regardless of tumor sidedness (left versus right),” the researchers noted.
Negative hyperselection represents a paradigm shift in the management of metastatic colorectal cancer, moving towards a more personalized and precise therapeutic approach. By meticulously excluding patients with genetic alterations associated with resistance to anti-EGFR therapies, clinicians can optimize treatment efficacy and improve patient outcomes. Further, comprehensive molecular profiling, including baseline and ongoing ctDNA analysis during treatment, serves as a critical tool in guiding treatment decisions.
- Stahler A, Kind AJ, Sers C, et al. Negative Hyperselection of Resistance Mutations for Panitumumab Maintenance in RAS Wild-Type Metastatic Colorectal Cancer (PanaMa Phase II Trial, AIO KRK 0212). Clin Cancer Res. 2024 Apr 1;30(7):1256-1263. doi: 10.1158/1078-0432.CCR-23-3023. PMID: 38289994.
- Turabi K, Klute K, Radhakrishnan P. Decoding the Dynamics of Circulating Tumor DNA in Liquid Biopsies. Cancers (Basel). 2024 Jul 1;16(13):2432. doi: 10.3390/cancers16132432.
- Shitara K, Muro K, Watanabe J, et al. Baseline ctDNA gene alterations as a biomarker of survival after panitumumab and chemotherapy in metastatic colorectal cancer. Nat Med. 2024 Mar;30(3):730-739. doi: 10.1038/s41591-023-02791-w. Epub 2024 Feb 12.
- Watanabe J, Muro K, Shitara K, et al. Panitumumab vs Bevacizumab Added to Standard First-line Chemotherapy and Overall Survival Among Patients With RAS Wild-type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA. 2023 Apr 18;329(15):1271-1282. doi: 10.1001/jama.2023.4428. Erratum in: JAMA. 2023 Jun 27;329(24):2196.
