DURHAM, NC—Bruton tyrosine kinase inhibitors are commonly prescribed for patients with mild to moderate renal impairment who have chronic lymphocytic leukemia (CLL), but whether the potential benefits of their use outweigh the risks in patients with severe renal dysfunction has been unclear.
A reluctance to use Bruton tyrosine kinase (BTK) inhibitors in patients with severely impaired kidney function arises from both a lack of clinical studies that include this patient population and a consequent silence on dosing from the U.S. Food and Drug Administration, as well as the association of the most commonly prescribed BTK, ibrutinib, with kidney injury and exacerbation of kidney dysfunction as a result of off-target effects.
BTK inhibitors have proven to be a powerful tool in CLL treatment and have replaced chemoimmunotherapy as the preferred first-line treatment. BTK inhibitors bind to the BTK enzyme and block a signaling cascade that leads to the proliferation of malignant (and normal) B cells. CLL, the most common type of adult leukemia, accounts for as much as 30% of all leukemias in the U.S. It is an indolent B-cell lymphoma characterized by monoclonal B cell proliferation.
Researchers at the Durham VAMC and the VA’s National Oncology Program looked to the VHA Corporate Data Warehouse to better understand the outcomes for veterans with CLL and severe renal dysfunction who were prescribed BTK inhibitors. They presented their results at the 65th American Society of Hematology Annual Meeting in San Diego on Dec. 11.
Across 68 VAMCs, they found 115 veterans with CLL who received a BTK inhibitor and, at some point in their treatment, had an estimated glomerular filtration rate (eGFR) of less than 30 ml/min/1.73 m2, their definition of severe renal dysfunction, between 2013 and 2022.1
Liu L, Pant N, Wang M, Grandhi N, Proskuriakova E, Janakiram M, Thomas T, Sanfilippo KM, Colditz G, Friedman DR, Schoen MW, Change SH. Monoclonal Gammopathy of Undetermined Significance and Risk of Cardiovascular Disease in US Veterans. Abstract 4773. ASH 2023. December 11, 2023.
Just under two-thirds (64%) of the patients had severe kidney impairment at the start of treatment, and 18% required dialysis at that point. The other 36% experienced increasing renal dysfunction during the course of treatment. Nearly all patients (98%) were male, a function of both the VA’s patient population and the higher incidence and more malignant course of CLL in men. The median age was 74 and the median duration of follow-up from the initiation of therapy was 26 months (range, 0.5-94).
The veterans received several BTK inhibitors, with the largest number receiving ibrutinib (103), followed by the second-generation and more selective BTK inhibitors acalabrutinib (18) and zanubrutinib (3). While 76% of the lines of BTK inhibitors started at the FDA-label dose, 37% required dose reduction, largely in response to toxicity. Discontinuation for toxicity was highest for ibrutinib (50%) compared to 39% for acalabrutinib and 33% for zanubrutinib. Other common reasons for discontinuation included infection, bleeding, arrhythmia and disease progression.
Among the ibrutinib patients, the median progression-free survival (PFS) was 45 months, and the overall survival was 49 months. Cirrhosis was associated with a more than sixfold reduction in progression-free survival (HR 6.36, 95% CI 1.45-19.76), while heart failure had a fourfold reduction (HR 4.20, 95% CI 1.82-9.47). Notably, severity of renal failure was not as impactful, with an 85% association with shorter PFS (HR 1.85, 95% CI 1.21-2.88). In multivariate modeling only heart failure and cirrhosis demonstrated a prognostic impact on overall survival.
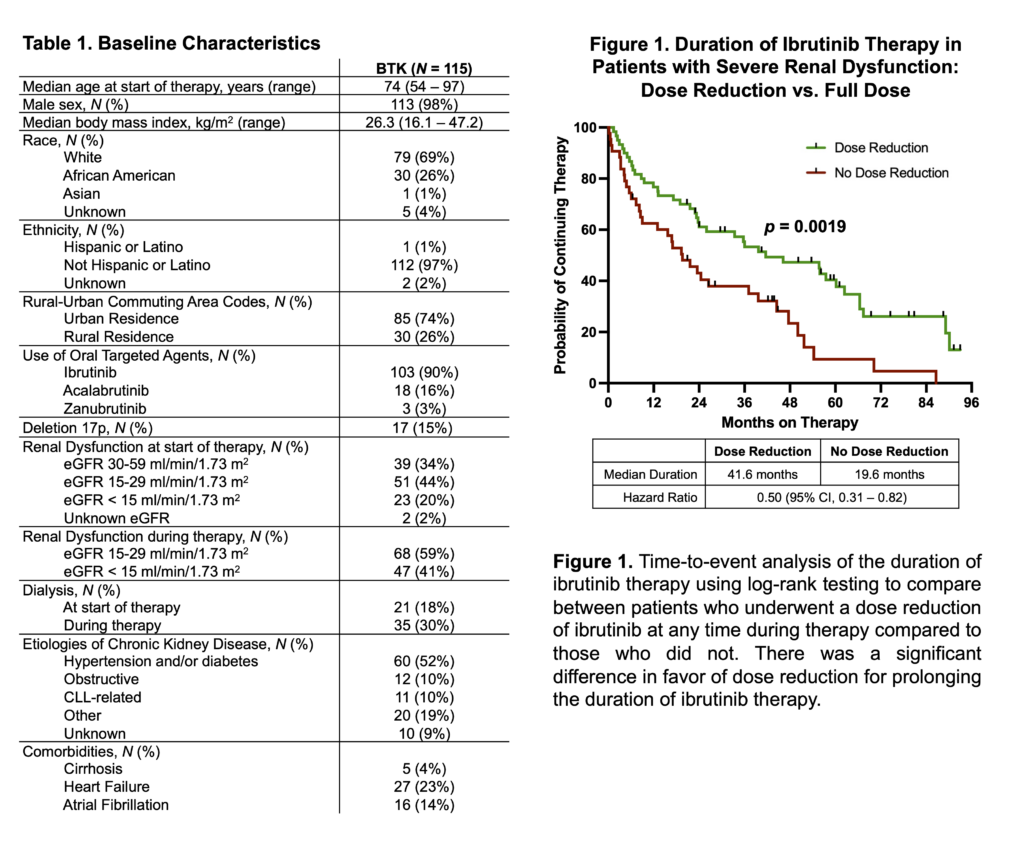
Ibrutinib therapy lasted a median of 33 months. Dose reductions at the start or during the course of treatment were associated with increased length of therapy but did not significantly reduce the response to ibrutinib or worsen survival. Half of the patients receiving ibrutinib experienced progression of renal dysfunction, as did 44% of those receiving acalabrutinib but none of the patients on zanubrutinib. No patients experienced improvement in kidney function after treatment with a BTK inhibitor, including among the 11 patients with CLL-related kidney impairment.
Based on these findings, the researchers concluded that BTK inhibitors offered a “reasonable therapeutic option for patients with CLL and severe renal impairment, even for older adults and patients on dialysis. They cautioned that patients should be closely monitored because of the risk of deterioration of kidney function and noted that dose modification could improve tolerability without impairing efficacy.
1Lin C, Anderson CE, Scobie MR, Rodgers TD, Kelley MJ, Friedman DR. Tolerability and Outcomes of Bruton Tyrosine Kinase Inhibitors for Chronic lymphocytic Leukemia in Patients with Severe Renal Dysfunction. Abstract 4648. ASH 2023. December 11, 2023.


