CHICAGO — Traditionally, treatment for colon cancer has been based primarily on the stage, but other issues—especially location—are becoming increasingly important.
According to the American Cancer Society, patients whose cancer hasn’t metastasized usually have surgery as the first-line treatment, but may have adjuvant treatment with chemotherapy for several months.
By Stage IV, colon cancer has spread from the colon to distant organs and tissues, usually the liver but also the lungs, brain, peritoneum (the lining of the abdominal cavity) or to distant lymph nodes. At that point, according to the cancer society, surgery is unlikely, and patients usually are treated with chemotherapy Th and/or targeted therapies, most likely to include one or more of the following:
- FOLFOX: leucovorin, 5-FU, and oxaliplatin (Eloxatin)
- FOLFIRI: leucovorin, 5-FU, and irinotecan (Camptosar)
- CAPEOX or CAPOX: capecitabine (Xeloda) and oxaliplatin
- FOLFOXIRI: leucovorin, 5-FU, oxaliplatin, and irinotecan
- One of the above combinations, plus either a drug that targets VEGF, (bevacizumab [Avastin], ziv-aflibercept [Zaltrap], or ramucirumab [Cyramza]) or a drug that targets EGFR (cetuximab [Erbitux] or panitumumab [Vectibix])
- 5-FU and leucovorin, with or without a targeted drug
- Capecitabine, with or without a targeted drug
- Irinotecan, with or without a targeted drug
- Cetuximab alone
- Panitumumab alone
- Regorafenib (Stivarga) alone
- Trifluridine and tipiracil (Lonsurf)
For patients with cancer cell changes in genes or proteins, targeted therapy drugs might be an option. The ACS advised that might include drugs that target blood vessel formation, such as vascular endothelial growth factor (VEGF), halting their action. In other cases, drugs targeting epidermal growth factor receptor (EGFR) are used. Limited research has been done on which type works better in which situation.
Tumor Location
Emerging research on the importance of tumor location is affecting some of those regimens, however. “Primary tumor sidedness (PTS) is an independent prognostic factor in metastatic CRC,” wrote the authors of a VA study published in Cancer Medicine. “PTS is also a predictive factor for response to EGFR inhibition in stage IV CRC, and laterality has been incorporated in the current version of National Comprehensive Cancer Network (NCCN) guidelines as a surrogate for response.”1
The study pointed out that past research on FOLFOX/FOLFIRI in combination with bevacizumab versus cetuximab found that right‐sided colon cancer (RCC) benefits less from cetuximab than left‐sided colon cancer (LCC).
“Embryologically, the right colon is derived from the midgut, while the left colon arises from the hindgut suggesting varied tumor biology,” the researchers added. “Hence, tumors arising from different embryological states are associated with distinct genetic drivers (RCC: BRAF mutation, MMRd, CpG island methylator phenotype CIMP vs. LCC: chromosomal instability, KRAS mutation, APC mutations), and ultimately different responses to systemic therapies.”
That study determined that, in early onset colorectal cancer, which occurs before age 50, patients with RCC have significantly worse survival than LCC in the metastatic setting (1‐year OS‐RCC: 59.84% vs. LCC: 73.23%; p = 0.0086). In the non-metastatic setting, however, they found no statistically significant difference in 5‐year overall survival at any stage.
New research from the Phase 3 PARADIGM trial could prove to be practice-changing, however, for treatment of some types of left-sided colorectal cancer. Early results were presented at the 2022 American Society of Clinical Oncology meeting in Chicago.
PARADIGM was the first prospective trial to test the superiority of panitumumab(PAN) vs. bevacizumab (BEV) combined with standard doublet first-line chemotherapy—mFOLFOX6—for patients with RAS wild-type (WT) metastatic colorectal cancer and left-sided primary tumors.2
The open-label, multi-center trial was conducted in Japan and randomly selected patients with chemotherapy-naive RAS WT metastatic colorectal cancer to PAN + mFOLFOX6 or BEV + mFOLFOX6.
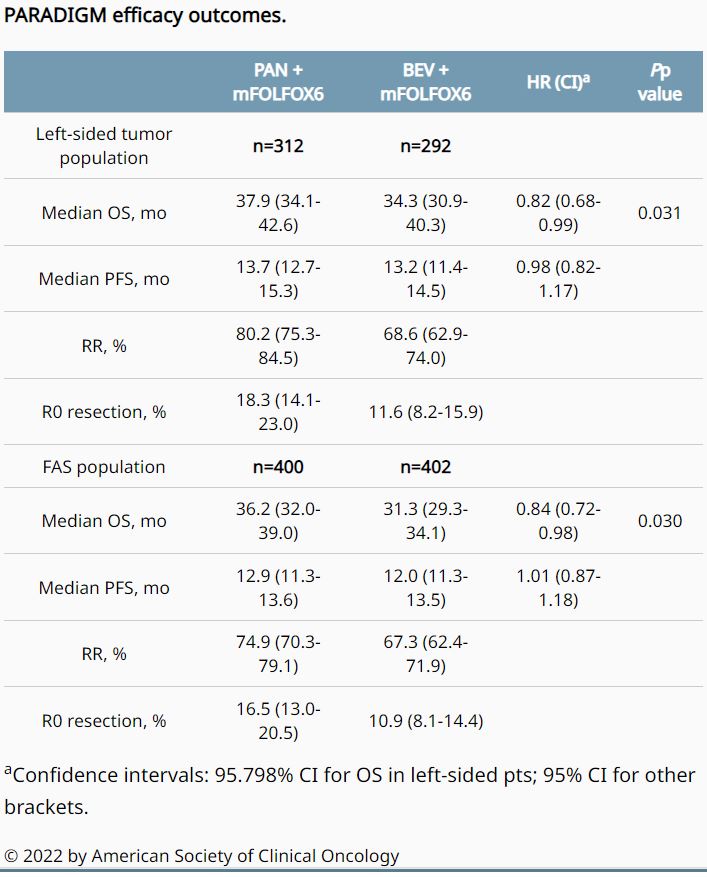
Researchers randomized 823 patients from May 2015 to June 2017, with 400 patients ultimately receiving PAN and 402 patients receiving BEV in the full-analysis set (FAS) population. Most of the patients, 312 and 292, respectively, had left-sided primary tumors.
The study team analyzed overall survival (OS) after 448 OS events in left-sided patients, with a median follow-up of 61 months. Results indicated that PAN significantly improved OS vs. BEV in both populations: left-sided (HR, 0.82; 95.798% CI, 0.68-0.99, p = 0.031, which crossed the boundary of significance [0.042]), and FAS (HR, 0.84; 95% CI, 0.72-0.98; p = 0.030, with < 0.05 as the boundary).
Researchers reported at ASCO that progression-free survival was comparable between treatment groups, but RR and R0 resection rates were higher with PAN compared with BEV. The hazard ratio for overall survival in the right-sided population was 1.09, they added, and no new safety signal was observed.
“PAN significantly improved OS vs. BEV in combination with mFOLFOX6 in patients with RAS WT and left-sided mCRC, establishing a standard first-line combination regimen for this population,” the authors concluded.
In a revised version from November, the authors wrote that first-line chemotherapy combined with panitumumab significantly improved overall survival compared with first-line chemotherapy combined with bevacizumab among patients with left-sided tumors (median overall survival, 37.9 months vs. 34.3 months; hazard ratio [HR] for death, 0.82), and in the overall population (median overall survival, 36.2 vs. 31.3 months; HR for death, 0.84).
The PARADIGM study was the first to compare an anti-EGFR antibody, panitumumab, with an anti-VEGF antibody, bevacizumab, when added to standard chemotherapy for patients with RAS wild-type disease and left-sided primary tumor. It followed two other trials, CALGB 80405 and the European FIRE-3, that sought to determine whether first-line chemotherapy should be combined with anti-EGFR or anti-VEGF antibodies in combination with the FOLFOX chemotherapy protocol, but looked at a more limited group of patients—those with left-sided colon tumors and without mutations in the RAS genes (KRAS and NRAS wild-type).
- Azar I, Al Masalmeh N, Esfandiarifard S, Virk G, Kiwan W, Frank Shields A, Mehdi S, Philip PA. The impact of primary tumor sidedness on survival in early-onset colorectal cancer by stage: A National Veterans Affairs retrospective analysis. Cancer Med. 2021 May;10(9):2987-2995. doi: 10.1002/cam4.3757. Epub 2021 Apr 2. PMID: 33797856; PMCID: PMC8085929.
- Yoshino T, Eatanabe J, Shitara K, Yasul H, et. al. Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Results from the phase 3 PARADIGM trial. .J Clin Oncol. 2022. 40, no. 17_suppl (June 10, 2022) LBA1-LBA1. Published online June 08, 2022.