Click to Enlarge: Source: Shift in First-Line Therapies for United States Veterans Health Administration Patients with Chronic
Lymphocytic Leukemia from 2014 to 2021. Abstract 4451. 2022 ASH Annual Meeting. Dec. 12, 2022.
SAN ANTONIO — For years, chemotherapy provided the only hope for patients with chronic lymphocytic leukemia (CLL). With the advent of Bruton tyrosine kinase (BTK) inhibitors nearly a decade ago, a number of more targeted therapies became available, many with fewer adverse effects and better outcomes than chemotherapy regimens such as chlorambucil, bendamustine and rituximab, CD20, fludarabine/cyclophosphamide/rituximab and other combinations.
Distribution of anticancer drugs to patients often takes years as evidence of effectiveness mounts, guidelines change, costs come down and payers approve treatments and, even then, some patients will be unable to access them because of high copayments or lack of insurance. In a system where several of the common impediments to adoption do not apply, how quickly do new therapies reach patients?
Kana Tai Lucero, MD, of the South Texas Veterans Health Care System in San Antonio and her colleagues analyzed records of 4,473 veterans treated for CLL at any VA facility between Jan. 1, 2014, and Dec. 31, 2021, to find out. The largest healthcare provider in the United States, the VA is a single-payer system that offers equal access to medications to veterans, eliminating issues of insurance coverage and financial burden to patients. The VA provides an unusually rich setting for analysis of CLL treatment as well, as it predominantly serves an older male population, precisely the demographic most affected by the disease.
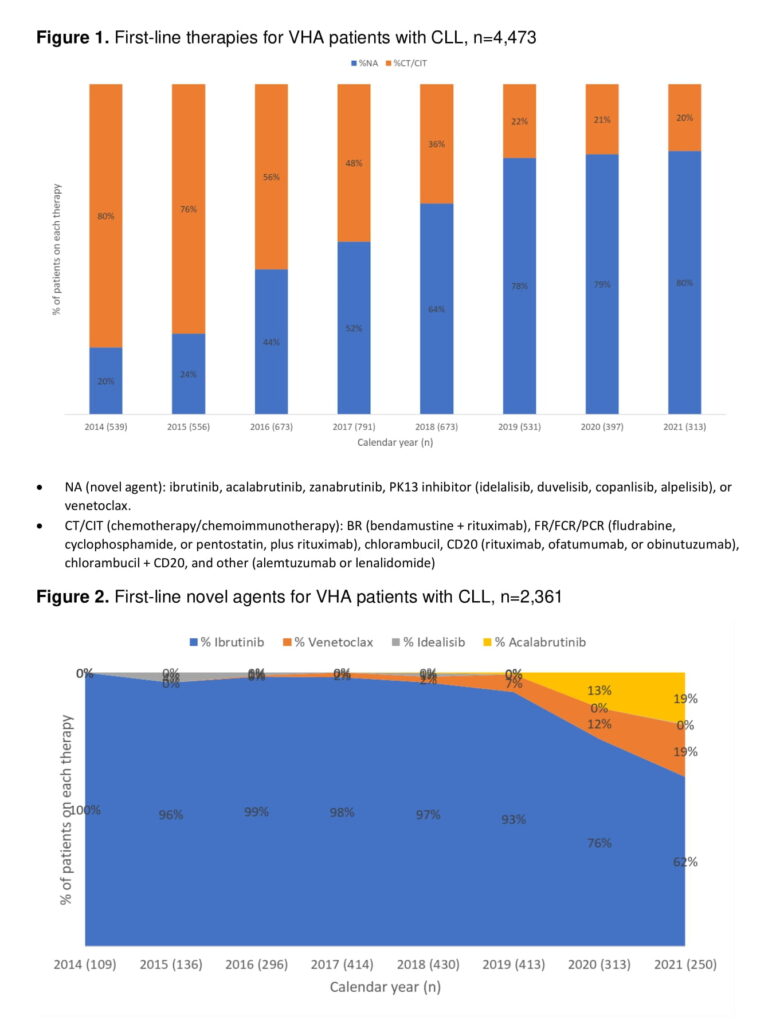
In a presentation at the 64th American Society of Hematology Annual Meeting in held Dec 10-13 in New Orleans, Lucero revealed that in 2014, the year ibrutinib, the first BTK inhibitor, was approved for CLL, novel agents accounted for 20% of first-line therapies for VA patients with CLL. By 2016, novel agents accounted for 44% of first-line therapies and became the majority of treatments (52%) in 2017. The novel agents—BTK inhibitors ibrutinib, acalabrutinib, and zanubrutinib; PK13 inhibitors idelalisib, duvelisib, copanlisib, and alpelisib; and venetoclax—represented nearly two-thirds of first-line therapies in 2018. Use of the newer therapies leveled off in 2019 at 78% and grew by 1% in subsequent years.1
Ibrutinib accounted for 90% of the novel agents (NAs) used as first-line therapies throughout the study period and for nearly 100% of NAs until 2018, when it started dropping with the U.S. Food and Drug approval of second-generation BTKs and other therapies. By 2021, ibrutinib was used in the first line in 62% of veterans. Venetoclax use rose from 2% in 2018 to 19% in 2021 and acalabrutinib increased from 0% in 2019, the year it was approved for use in CLL, to 19% two years later. Idealisib use peaked at 4% in 2015, then declined to between 0% and 1% for the remainder of the study period.
“There has been a major shift in 1L treatments with NAs outpacing chemotherapy-based regimens from 2017-2021,” said the researchers. “The choice of first-line therapy continues to evolve as options with better side effect profiles become available and new combinations emerge that stimulate deeper response or require shorter treatment duration.”
- Lucero KT, Frei CR, Ryan KJ, Obodozie-Ofoegbu OO, Moore AM, Teng C, Jones X, Nooruddin Z. Shift in First-Line Therapies for United States Veterans Health Administration Patients with Chronic Lymphocytic Leukemia from 2014 to 2021. Abstract 4451. 2022 ASH Annual Meeting. Dec. 12, 2022.

