Study Used Data from VA, Elsewhere
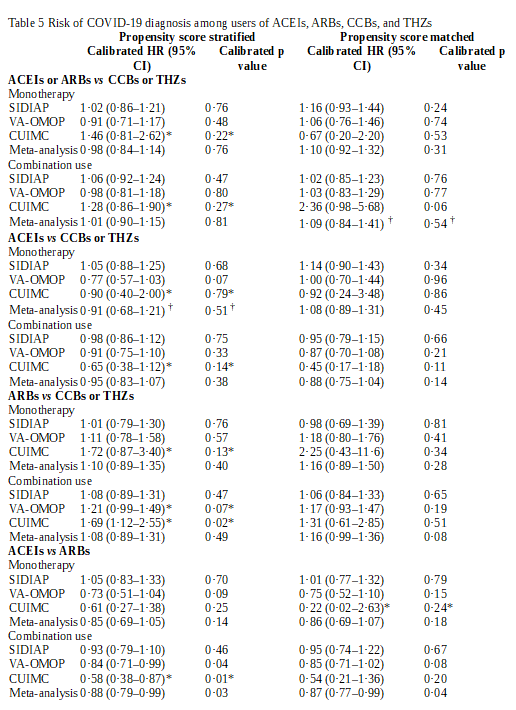
Click To Enlarge: ACEI=angiotensin-converting enzyme inhibitor. ARB=angiotensin receptor blocker. CCB=calcium channel blocker. CUIMC=Columbia University Irving Medical Center data warehouse. HR=hazard ratio. SIDIAP=Information Systems for Research in Primary Care. THZ=thiazide or thiazide-like diuretic. VA-OMOP=US Department of Veterans Affairs Observational Medical Outcomes Partnership.
* Data source entries do not pass study diagnostics and not included in the meta-analytic estimate.
† Entries return heterogeneity ( I 2) values of more than 40%.
LOS ANGELES – In the chaotic days when the COVID-19 pandemic first began to affect the United States, healthcare professionals were barraged with questions about certain blood pressure medications and whether they increased infection risk for patients using them.
Now, the most comprehensive study yet uses data from the VA and other international healthcare systems to find no increased risk of COVID-19 diagnosis, hospitalization, or subsequent complications for users of either angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) among an international cohort of more than 1.1 million patients using antihypertensives.
The report in The Lancet Digital Health bolsters regulatory and clinical recommendations that patients should not discontinue ACE inhibitor or ARB therapy due to concerns of increased COVID-19 risk.1
“People with hypertension have worse COVID-19 outcomes, and there remains speculation that some anti-hypertensive medications may be detrimental,” explained corresponding author Marc A. Suchard, MD, PhD, a professor at UCLA. “The clear answer is that ACE inhibitors and ARBs pose no increased risk as compared to other treatments.”
For the international, open science, cohort analysis, Observational Health Data Sciences and Informatics (OHDSI) researchers used electronic health records from and the VA’s Observational Medical Outcomes Partnership [VA-OMOP]) — as well as additional data from Spain (Information Systems for Research in Primary Care [SIDIAP]) and the United States(Columbia University Irving Medical Center data warehouse [CUIMC] — to identify adults with at least one prescription for ACEIs and ARBs (target cohort) or calcium channel blockers (CCBs) and thiazide or thiazide-like diuretics (THZs; comparator cohort) between Nov 1, 2019, and Jan 31, 2020.
The study defined users separately as receiving either monotherapy with these four drug classes, or monotherapy or combination therapy (combination use) with other antihypertensive medications.
Researchers assessed four outcomes:
- COVID-19 diagnosis;
- hospital admission with COVID-19;
- hospital admission with pneumonia; and
- hospital admission with pneumonia, acute respiratory distress syndrome, acute kidney injury, or sepsis.
Results indicate that, among nearly 1.4 million antihypertensive users, no association was observed between COVID-19 diagnosis and exposure to ACEI or ARB monotherapy vs. CCB or THZ monotherapy (calibrated hazard ratio [HR] 0·98, 95% CI 0·84–1·14) or combination use exposure (1·01, 0·90–1·15).
Researchers add that ACEIs alone showed no relative risk difference when compared with CCB or THZ monotherapy (HR 0·91, 95% CI 0·68–1·21; with heterogeneity of >40%) or combination use (0·95, 0·83–1·07).
On the other hand, “directly comparing ACEIs with ARBs demonstrated a moderately lower risk with ACEIs, which was significant with combination use (HR 0·88, 95% CI 0·79–0·99) and non-significant for monotherapy (0·85, 0·69–1·05),’ the authors note.
The study observed no significant difference between drug classes for risk of hospital admission with COVID-19, hospital admission with pneumonia, or hospital admission with pneumonia, acute respiratory distress syndrome, acute kidney injury, or sepsis across all comparisons.
“No clinically significant increased risk of COVID-19 diagnosis or hospital admission-related outcomes associated with ACEI or ARB use was observed, suggesting users should not discontinue or change their treatment to decrease their risk of COVID-19,” researchers conclude.
“Based on our results, if there is a risk difference, it’s marginal and would be very challenging to further refine outside such a large-scale international study,” Suchard added.
“By comparing people exposed to ACE inhibitor and ARBs against people taking other antihypertensives, either alone or in combination, using two methods across three database the study generated 1280 comparisons to assess the safety of these drugs, producing highly consistent results,” advised lead author Daniel Morales, BMSc MBChB PhD, Wellcome Trust Clinical Research.
The authors pointed out that studies assessing the risk of COVID-19 among ACEI or ARB users have been published from Italy, Spain, the UK, and the US. They also concluded, after adjustment for the higher prevalence of cardiovascular conditions in patients with COVID-19, that ACEI and ARB use was not associated with an increased risk of COVID-19 diagnosis, according to the report.
The problem, however, is that the case-control studies included only a limited number of covariates for model adjustment, according to the researchers, who pointed out, “We identified only two studies that compared the risk of COVID-19 susceptibility in ACEI or ARB users with an active comparator.”
“In this context, comparing patients with similarly indicated treatments is crucial for reducing the risk of bias resulting from confounding by indication (eg, hypertension), in which the absence of treatment indicates either too mild a disease to warrant pharmacological treatment (eg, mild hypertension under control with lifestyle and diet changes), the presence of contraindications, or extreme frailty precluding the use of preventive medicines (eg, at the end of life),” they explained.
The bottom line, according to the authors, is that their results “stand in agreement with regulatory and clinical society advice that ACEI and ARB therapy should be continued in light of COVID-19. Furthermore, the marginal difference between ACEIs and ARBs does not warrant class switching to reduce COVID-19 susceptibility.”
-
Morales DR, Conover MM, You SC, Pratt N, et. Al. Kostka K, Duarte-Salles T, Fernández-Bertolín S, Aragón M, DuVall SL, Lynch K, Falconer T, Renin-angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis. Lancet Digit Health. 2020 Dec 17:S2589-7500(20)30289-2. doi: 10.1016/S2589-7500(20)30289-2. Epub ahead of print. PMID: 33342753.


