Mary Jo Pugh, PhD, RN
Researchers Determine That Risks Peaks Prior to AED Initiation
By Annette M. Boyle
SAN ANTONIO—In 2008, the U.S. Food and Drug Administration issued an alert about increased risk for suicidal ideation and behavior for patients taking anti-epileptic drugs (AEDs). Since then, a number of studies have tried to determine whether the signals detected in clinical trials actually indicated an increased risk of suicidality and whether the class of drug or condition being treated by the medications affected the risk.
A recent study by VA researchers suggests that the FDA alert may have been triggered by factors unrelated to exposure to anti-epileptics.1
The team analyzed VA health system data of veterans who had been deployed to Iraq or Afghanistan and received care at the VA. Of that cohort, about 15% or 90,000 veterans receive an anti-epileptic medication each year, according to lead author Mary Jo Pugh, PhD, RN, of the University of Utah School of Medicine, formerly of the South Texas Veterans Health Care System.
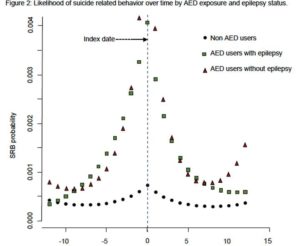
The study included those who received their first AED prescription in 2013 and 2014 and identified suicide-related behavior (SRB) that occurred 12 months before and after starting AEDs. The analysis demonstrated that the probability of SRB diagnosis increased prior to the initial prescription of AEDs and then dropped. A similar trajectory was seen in non-AED users over the same time period, but that group was 94% less likely to exhibit suicide-related behaviors. The results persisted when adjusted for sociodemographic factors, mental health comorbidities and epilepsy status.
“The most important finding from this study was that suicide-related behavior peaked prior to the initiation of AEDs and rapidly decreased after the drug was prescribed. This suggests a strong possibility that the relationship of AED and SRB is one of residual confounding and may not be due to medication exposure,” Pugh told U.S. Medicine.
In an earlier study published in Neurology, the research team had found the same type of relationship for older veterans who received AEDs.2 The researchers concluded in that study that “rather than highlighting the association between SRB and AED exposure, these data suggest that individuals who received the AED were at greater risk of SRB both before and after initiation of AED, even after controlling for psychiatric comorbidity.”
Based on the results of those studies, Pugh recommended that clinicians share all the information available about AEDs and suicidality with patients. “Patients should know that the FDA identified AEDs as associated with suicide, however, data from the VA in different subpopulations of veterans suggests that the peak of SRB occurs prior to exposure, and may be due to the types of conditions for which AEDs are used. Moreover, after AED treatment is initiated, SRB rapidly decreases,” she noted.
The FDA did not consider the possible 80% increase in suicidality—which was detected in their analysis of adverse events and reported in connection with 199 randomized clinical trials—sufficient to warrant a black box warning. Its meta-analysis combined data concerning 11 AEDs including carbamazepine, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, valproate, and zonisamide. The VA studies also included clobazam, clonazepam, eslicarbazepine acetate, ethosuximide, locasamide, perampanel, phenobarbiatal, phenytoin, primidone, retigabine, rufinamide and vigabatrin.
Other analyses of suicide-related behaviors and AEDs have come to a variety of conclusions. Some studies found that certain medications may increase the risk more than others, while others suggested that psychological comorbidities explained the increased risk. Yet others found no correlation.
No Other Options
Even if AEDs modestly increase suicidal behaviors, the risk of not taking them is often much greater, Pugh said. “For patients with epilepsy, there are, in general, no other options for treatment. As such, the risk for patients with epilepsy is substantial. Uncontrolled epilepsy is associated with a host of adverse effects, including sudden unexplained death in epilepsy.”
Anti-epileptics might also be prescribed to treat a number of conditions other than epilepsy, including headache, bipolar disorder, chronic neuropathic pain and depression. While patients with these conditions have alternative therapies available, “anti-epileptic medications are typically used only after the failure of more traditional approaches” such as triptans for migraine, lithium or atypical antipsychotics for bipolar disorder and antidepressants or opioids for chronic pain, she said. “Thus, these patients are more resistant to other treatment modalities and the risk of not treating is likely as severe as treating with AEDs.”
The two VA studies indicate that suicidality is highest shortly before treatment commences and, once patients are treated, the risk for suicidality declines substantially, suggesting that treatment leads to improvements, Pugh added.
Still, she advised a conservative approach to further reduce risk. “While there are no mandates of guidelines that endorse screening for depression and/or suicidality for individuals who eventually received AEDs, my personal belief is that there is no harm (and may be benefit to the patient) by doing so,” Pugh noted. Many studies have documented a high rate of comorbidity between epilepsy and both suicidality and major depression.
“Given that the majority of conditions AEDs are used for are related to depression and/or suicide, and the FDA statement that AEDs are suicidogenic, individuals who receive AEDs may be considered a high-risk population.”
- Pugh MJ, Sagiraju H, Wang CP, Hamid H. A Temporal Evaluation of Anti-epileptic Drugs and Suicidality: Is It the Medication or the Comorbidity. American Epilepsy Society Annual Meeting 2017. Abst. 2.294.
- Pugh MJ, Hesdorffer D, Wang CP, Amuan ME, Tabares JV, Finley EP, Cramer JA, Kanner AM, Bryan CJ. Temporal trends in new exposure to anti-epileptic drug monotherapy and suicide-related behavior. Neurology. 2013 Nov 26;81(22):1900-6.