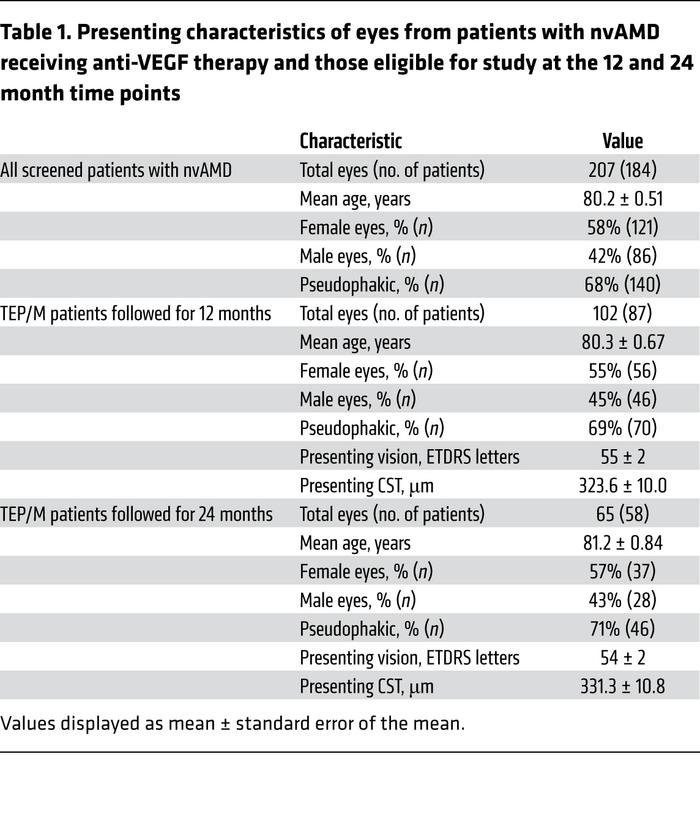
Click To Enlarge: Presenting characteristics of eyes from patients with nvAMD receiving anti-VEGF therapy and those eligible for study at the 12 and 24 month time points
BALTIMORE, MD — Age-related macular degeneration (AMD) is the leading cause of severe vision loss in individuals over the age of 50 years, which is especially significant for U.S. veterans. Male veterans had a median age of 65 in 2017, with female veterans somewhat younger at 51.
Choroidal neovascularization (CNV) causes “wet”’ AMD and is responsible for about 90% of cases of severe vision loss due to AMD.
A new study from Johns Hopkins University School of Medicine points out that, in an effort to reduce the treatment burden for patients with neovascular age-related macular degeneration (nvAMD), emerging therapies targeting vascular endothelial growth factor (VEGF) are being designed to extend the interval between treatments. While that can minimize the number of intraocular injections, the report in the Journal of Clinical Investigation cautions that it will be important to determine which patients will benefit from longer-acting agents.1
For the study, researchers treated 122 eyes with nvAMD in three consecutive monthly injections with currently available anti-VEGF therapies, followed by a treat-and-extend protocol. Patients whose symptoms remained well-controlled 12 weeks from their prior treatment were allowed to pause the injections and, instead, were switched to pro re nata (PRN) treatment, which was based on vision, clinical exam, and/or imaging studies. At the same time, proteomic analysis was performed on aqueous fluid to identify proteins that correlate with patients’ response to treatment.
“Such a test could let us tell patients early on how well they would do and when they might be able to stop,” explained co-author Akrit Sodhi , MD, PhD, associate professor of ophthalmology at the Johns Hopkins University School of Medicine and Wilmer Eye Institute.
The study team concludes that aqueous biomarkers “could help identify patients with nvAMD who may not require or benefit from long-term treatment with anti-VEGF therapy.”
By the end of the first year, 38 of 122 eyes (31%) had entered a treatment pause of 30 weeks or more. Still, 21 of 122 eyes (17%) failed extension and required monthly treatment at the end of that year.
Response to Treatment
Researchers report that proteomic analysis of aqueous fluid identified proteins that correlated with patients’ response to treatment, including proteins previously implicated in AMD pathogenesis.
“Interestingly, apolipoprotein-B100 (ApoB100), a principal component of drusen implicated in the progression of non-neovascular AMD, was increased in treated patients who required less frequent injections,” the authors advise. “ApoB100 expression was higher in AMD eyes compared with controls but was lower in eyes that develop choroidal neovascularization (CNV), consistent with a protective role.”
They also point out that, in animal studies, mice over-expressing ApoB100 were partially protected from laser-induced CNV.
“Across the board, the patients who could enter a treatment pause did the best even though they were receiving no anti-VEGF drugs. They had better visual acuity, better gain of vision and less fluid in their retina,” Sodhi advised.
Background information in the study points out, “The recent introduction of therapies targeting vascular endothelial growth factor (VEGF), has had a remarkable impact on patients with nvAMD.”
Researchers expressed concern that anti-VEGF therapies are provided indefinitely, which is worrisome because of “the substantial economic and social burden of frequent clinic visits for elderly patients who often require assistance for transportation and mobility.:
The study calls the “sustainability of indefinite intraocular injections with anti-VEGF therapy” a “reasonable concern.” The authors also advise that clinicians have sought to reduce the number of treatments with currently available therapies, which lowers necessary visits and potential adverse events.
The authors point out that patients with nvAMD “are not a homogeneous population, but instead are comprised of three distinct subgroups: patients who respond inadequately despite monthly anti-VEGF therapy (q4 patients; approximately 20%); patients who respond to anti-VEGF therapy but require indefinite treatment (approximately 50%); and patients who can be weaned off treatment (q12+ patients; approximately 30%).”
Researchers further posit that “there are definable subgroups of patients with nvAMD who respond differently to anti-VEGF therapy. It is also not known whether specific anti-VEGF therapies may be more effective than others at weaning patients off therapy. The ability to wean patients off therapy may have a significant impact on the long-term cost-effectiveness of anti-VEGF therapies. These unanswered questions are under current investigation. This is particularly relevant given emerging ethical issues about the distribution of cost-effective versus most effective anti-VEGF therapies among patients with ocular neovascular disease.”
The authors also suggest that the ability to determine how patients will respond to anti-VEGF therapy will also be essential for deciding which patients will benefit from the next generation of longer-acting anti-VEGF therapies. Their findings demonstrate that aqueous biomarkers can help do that.
- Cao X, Sanchez JC, Dinabandhu A, Guo C, Patel TP, Yang Z, Hu MW, Chen L, Wang Y, Malik D, Jee K, Daoud YJ, Handa JT, Zhang H, Qian J, Montaner S, Sodhi A. Aqueous proteins help predict the response of patients with neovascular age-related macular degeneration to anti-VEGF therapy. J Clin Invest. 2022 Jan 18;132(2):e144469. doi: 10.1172/JCI144469. PMID: 34874918; PMCID: PMC8759792.

