ROCKVILLE, MD—Thanks to a new partnership between the National Cancer Institute and the VA, veterans with cancer will now have greater access to potentially lifesaving clinical trials.
The NCI and VA Interagency Group to Accelerate Trials Enrollment (NAVIGATE) is launching at 12 VA facilities across the country.
“Strategic partnerships, such as this one with the National Cancer Institute, allow VA to leverage the strengths of both organizations to the benefit of all stakeholders, especially our veterans,” said Peter O’Rourke, senior adviser to the secretary of the VA. “By increasing enrollment in these trials, VA and veterans will contribute to important cancer research—this will not only help our veterans but also advance cancer care for all Americans and people around the world.”
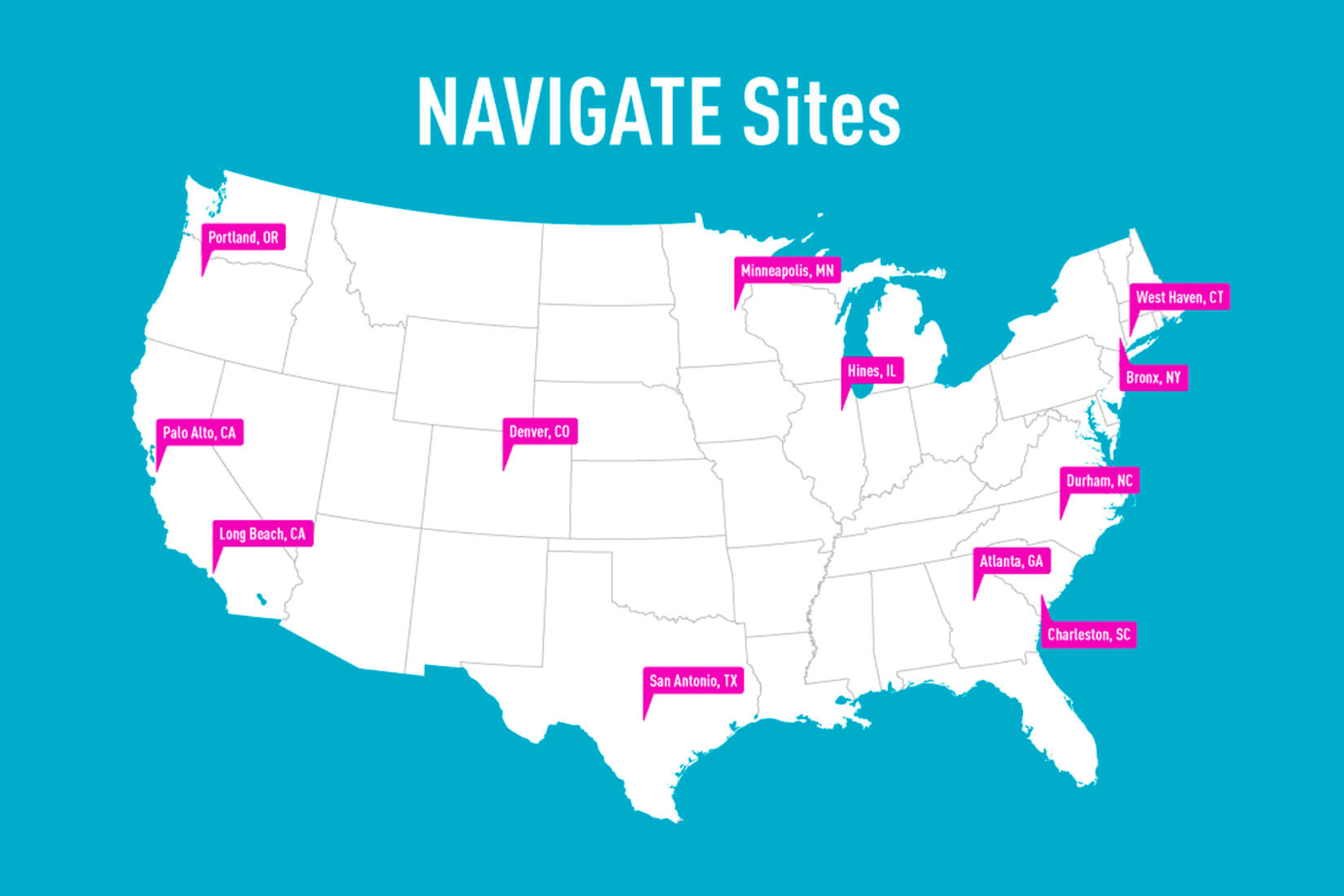
The sites involved in the VA program include Atlanta; Bronx, NY; Charleston, SC; Denver; Durham, NC; Hines, IL; Long Beach, CA; Minneapolis; Palo Alto, CA; Portland, OR; San Antonio; and West Haven, CT.
Activities for the entire network of sites will be coordinated in Boston.
“Since goals of NAVIGATE include ensuring communication across multiple VA medical centers on successes and barriers to conducting NCI clinical trials and to implement best practices for recruitment, the coordinating center will help facilitate these goals in addition to providing administrative and management support for the national network,” explained Grant D. Huang, MPH, PhD, director of the Cooperative Studies Program in the VA Office of Research and Development.
Boston already has a robust cancer clinical trials program operating through the VISN 1 Clinical Trials Network. Currently, the VISN has about 20 cancer clinical trials in the region and about an equal number in cardiology, mental health, maternity care, Gulf War Illness and other areas.
“The ability to coordinate activities involving the same team at the Boston Coordinating Center for NAVIGATE will help better communications and leveraging of opportunities and lessons,” Huang told U.S. Medicine.
NAVIGATE will provide funding to coordinate activities at the sites to meet administrative, regulatory, recruitment and other requirements for the trials. At the same time, the sites will work together to identify obstacles to enrollment within the VA and develop best practices to help other VA centers take advantage of NCI trials.
While increasing enrollment at the 12 sites will be the primary focus of the NAVIGATE program, “any VA medical enter currently has the capability to participate in NCI-funded trials on an individual basis,” Huang said.
Veterans Helping Others
The NCI and VA began discussing the partnership in 2016 to improve veteran access to clinical trials, reverse an overall decline in trial enrollment and advance the national Cancer Moonshot program. The program will help both agencies meet important aims. Through NAVIGATE, both organizations hope to increase participation of minorities in clinical trials.
“This agreement will not only provide veterans greater access to NCI clinical trials, it will enhance accrual to NCTN [National Clinical Trials Network] and NCORP [NCI Community Oncology Research Program] trials, resulting in more timely completion of these studies. This interagency collaboration will also work to help veterans overcome barriers they’ve faced trying to access clinical trials as part of their cancer care,” noted James H. Doroshow, MD, deputy director for Clinical and Translational Research at NCI.
On the VA side, “the partnership will allow the sharing of expertise and resources in their respective strengths to benefit veterans and the nation. In addition to access to trials and the opportunities they provide, veterans are given another opportunity to serve their country by contributing to nationally impactful research,” Huang said.
Even after their service, veterans often continue to look for opportunities to advance national goals and help others. Researchers in VISN 1 have found that veterans are willing to participate even in trials that do not offer a cure, such as the ONCOCYTE trial, which aims to identify serum biomarkers of lung cancer that can enable earlier diagnosis of disease.
“Veterans understand that not everyone benefits from clinical trials, but they think, ‘If I don’t help myself, at least I help other people,’” according to Herta H. Chao, MD, PhD, cancer clinical trial director and deputy chief of hematology and oncology for the West Haven VAMC. “Veterans are very altruistic.”
The VA also will help NCI collaborators navigate the VA healthcare system and understand the finer points of military culture, veteran concerns and specific challenges. NAVIGATE will help NCI investigators “connect various [VA} components as seamlessly as possible on a national level to benefit recruitment into NCI trials. It will leverage activities already being conducted by the VA Cooperative Studies Program for its VA-funded multi-site clinical trials and seek to adapt them into needs for NCI trials,” Huang explained.
Building stronger clinical trial infrastructure within the VA will enable veterans to participate in trials for experimental treatments, including precision-medicine therapies based on genetic profiles and new immunotherapies. Targeted therapies and immunotherapies have transformed care for many types of cancer in recent years, as they have replaced chemotherapy as first-line treatment. For other types of cancer, participation in clinical trials is the recommended first choice of treatment.
The VA already is contributing significantly to researchers’ understanding of the genetic basis of diseases through its pioneering Million Veteran Program, which is on target to create the world’s largest genomic database. Veterans who participate in the program volunteer to share their genetic material and other health information and to match it against their health records to provide insights into the links between genetics and health.

