SAN FRANCISCO—Although a recent study determined that screening veterans with cirrhosis for hepatocellular carcinoma did not reduce the risk of death associated with liver cancer, the VA has no plans to change its screening practices.
“The VA currently follows the American Association for the Study of Liver Diseases (AASLD) guidelines for HCC screening among patients with cirrhosis,” explained Maggie Chartier, PsyD, MPH, the VA’s deputy director of HIV, Hepatitis and Related Conditions and associate professor at the University of California, San Francisco. The AASLD recommends screening patients with cirrhosis for HCC using ultrasound (USS) with or without serum alpha fetoprotein measurement every six months.
Those recommendations mean that more than 60,000 veterans with cirrhosis should be screened twice a year, based on estimates in a new study on the cost of hepatocellular carcinoma among veterans.1 An estimated 85% to 95% of HCC patients have cirrhosis, though the malignancy can develop in patients with chronic hepatitis B infection without cirrhosis occurring first. More than 2000 new cases of HCC are diagnosed in veterans in VA care annually.
The AASLD guidelines based the screening recommendation on a meta-analysis of 38 observational studies with a total of 10,850 patients which found a pooled three-year survival rate of 50.8% among patients who received surveillance compared to only 27.9% among those who did not. Screening also doubled the likelihood that HCC would be detected at an early stage, when patients were eligible for more curative treatments.
Results from a recent VA study challenge the AASLD recommendations, however. VA researchers at the Puget Sound Health Care System and VA San Diego Healthcare System and others found that “screening patients with cirrhosis for HCC by USS, measurement of serum AFP, either test, or both tests was not associated with decreased HCC-related mortality.”2
The researchers conducted a matched case-control study using VA records. They matched 238 veterans with cirrhosis who died of hepatocellular carcinoma between Jan. 1, 2013, and Aug. 31, 2015 and had been in VA care for at least four years prior to the diagnosis of HCC to 238 patients with cirrhosis who did not die of HCC and had also received care through the VA for the four years before the matched case was diagnosed with HCC. Controls were matched by year of cirrhosis diagnosis, race and ethnicity, age, sex, etiology of cirrhosis, Model for End-Stage Liver Disease score and VA medical center where they received treatment. All ultrasound and AFP tests during the period were identified and evaluated to determine whether they were conducted for screening purposes or other reasons.
The researchers found that screening rates did not differ significantly between the group of patients who died of HCC and those who did not. Among those who died, the rate of screening by either ultrasound or serum AFP was actually slightly higher, 81.1%, compared to those who did not, 79.4%. The screening rates did not differ within one, two or three years of the date of HCC diagnosis.
Chartier told U.S. Medicine that a number of factors distinguished the VA study from those cited in the AASLD guidelines. “The difference is explained by the differences in study design, including different study populations and details about the indication for the tests that were performed to look for cancer. It is hard to tell which study design best presents data for use in establishing guidelines,” she noted. “Ultimately, a randomized controlled trial may be needed.”
Efficacy of Screening
The study authors agreed that additional research is required, and they did not conclude that physicians should disregard the AASLD recommendations. Instead, they encouraged “additional case-control studies to evaluate the efficacy of screening for HCC in other health care systems, in which available records are sufficiently detailed to enable identification of the indication for USS and AFP tests.”
Case-control studies eliminate much of the survival bias seen in the studies included in the AASLD guidelines, study co-author George Ioannou, MD, MS, FAASLD, told U.S. Medicine. “The case-control study design of screening effectiveness is not subject to lead time bias or length time bias. Previously published studies only employed cases (patients with liver cancer) and then attempted to compare the survival of those cases diagnosed by screening to the survival of those cases in whom the diagnosis was made as a result of symptomatic presentation.”
“This design is fundamentally flawed and subject to lead time bias and length time bias. Furthermore, the bias in all these studies is in the same direction: making the survival appear longer in the patients diagnosed by screening,” Ioannou said.
Demonstrating a reduction in cancer-related mortality associated with screening is “a tall hurdle to overcome,” he said, adding that “I am only aware of two or three cancer screening programs for any cancer that have ever convincingly demonstrated a substantial reduction in cancer mortality.”
Still, “until a better screening strategy is identified, we should all employ the current screening strategy recommended by the AASLD,” Ioannou said. He also recommended enrolling patients in clinical trials that are evaluating new screening strategies.
Reducing the burden associated with hepatocellular carcinoma could have significant impact on the VA. While the “total number of veterans diagnosed with HCC decreased from a high of 10,850 in 2014 to 8,633 today,” according to Chartier, the cost per patients is quite high.
A recent study of the cost of HCC in the VA determined that caring for patients with HCC costs $158,688 over a three-year period, while the same length of care for cirrhotic patients who do not develop HCC costs $69,010. Two-thirds of the additional $85,679 attributed to the malignancy largely arises from increased inpatient costs. Liver transplantation led to a three-year incremental cost of $422,007 compared to controls with cirrhosis and a $396,735 increase in costs compared to HCC patients who did not receive transplants.
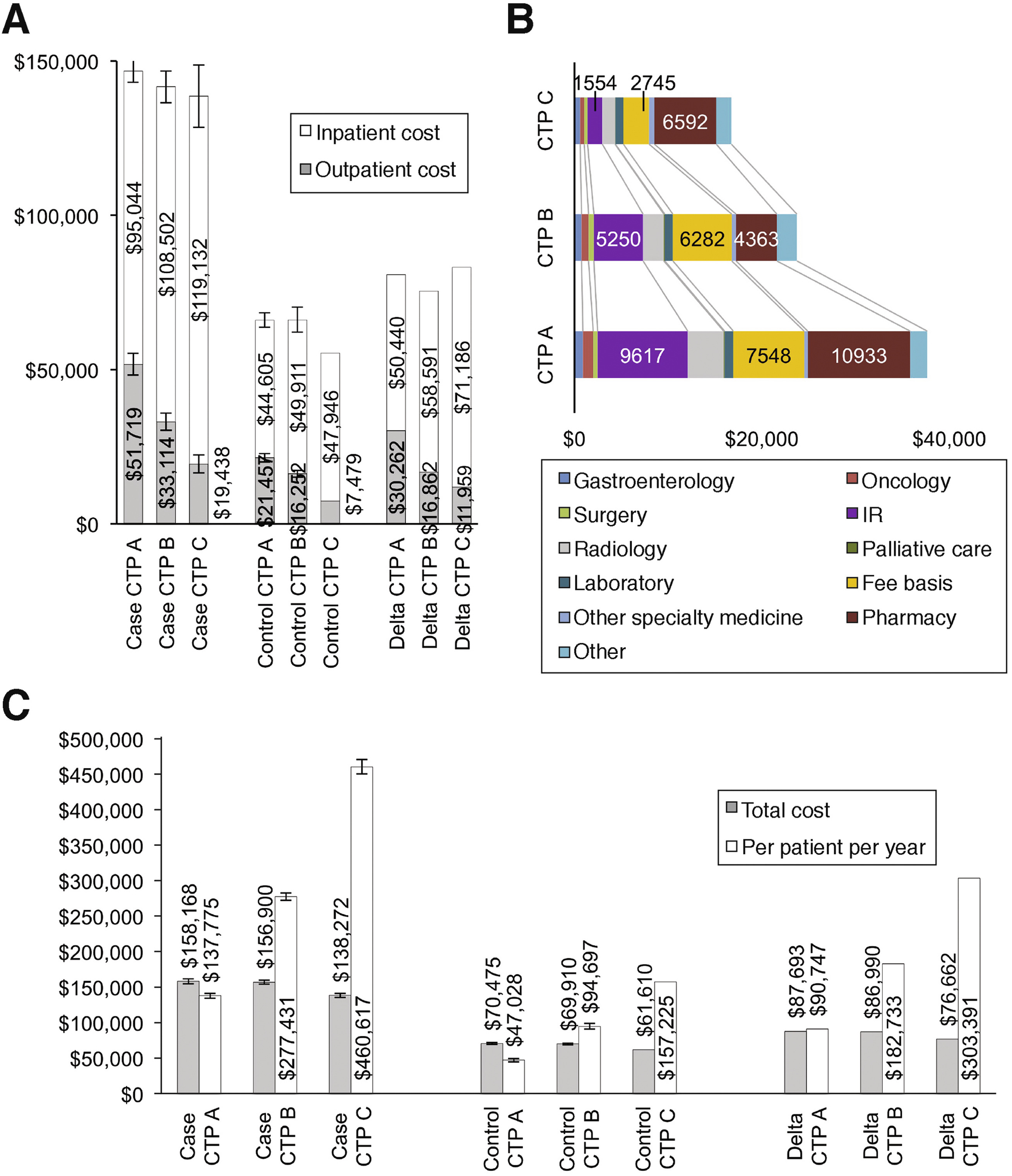
The study, led by David Kaplan, MD, of the Corporal Michael J. Crescenz VAMC in Philadelphia, involved researchers at multiple VAMCs and was published in Clinical Gastroenterology and Hepatology. The team compared the cost of care for 3,188 veterans with HCC to 12,722 veterans with cirrhosis matched to the cases by disease severity, age and comorbidities.
The VA’s success in treating veterans with hepatitis C has contributed significantly to the recent decline in the number of HCC cases at the VA from 10,850 in 2014 to 8,653 today. Chartier noted that viral hepatitis had accounted for 74.5% of all cases of HCC at the VA in 2014 and now the proportion of HCC attributable to viral hepatitis is 70.6%.
As hepatitis C declines, other factors are increasing, however, so whether the current drop in incident cases of HCC will be maintained remains an open question. According to the AASLD guidelines, “recent data have also shown that metabolic disorders—defined as nonalcoholic fatty liver disease (NAFLD) and the metabolic syndrome—contribute numerically more to the burden of HC than any other risk factor including HCV infection.”
About one-third of veterans might meet the definition of NAFLD, based on recent analysis of data from the Million Veteran Project, led by Marina Serper, MD, of the Corporal Michael J. Crescenz VAMC and the University of Pennsylvania Perelman School of Medicine, both in Philadelphia. The analysis excluded patients with alcohol misuse disorder or viral hepatitis.3
1. Kaplan DE, Chapko MK, Mehta R, Dai F, Skanderson M, Aytaman A, Baytarian M, D’Addeo K, Fox R, Hunt K, Pocha C, Valderrama A, Taddei TH; VOCAL Study Group. Healthcare Costs Related to Treatment of Hepatocellular Carcinoma Among Veterans With Cirrhosis in the United States. Clin Gastroenterol Hepatol. 2018 Jan;16(1):106-114.e5.
2. Moon AM, Weiss NS, Beste LA, Su F, Ho SB, Jin GY, Lowy E, Berry K, Ioannou GN. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology. 2018 Jul 5. pii: S0016-5085(18)34721-8.
3. Fulton, D. AASLD 2017 Conference Coverage. Video: Huge Database analysis affirms genes associated with NAFLD. Internal Medicine News. October 24, 2017.

