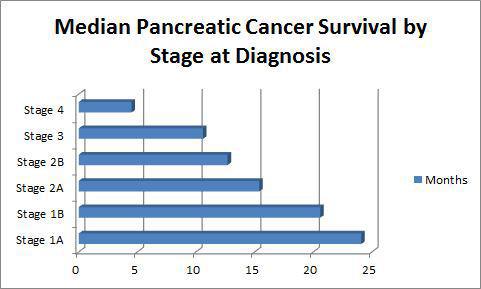
ALBANY, NY — While many other cancers have seen dramatic improvement in outcomes in the past 20 years, pancreatic cancer remains one of the deadliest malignancies, regardless of stage at diagnosis, with an overall five-year survival rate of only 8%, according to the American Cancer Society.1
The extremely high mortality rate has led to “therapeutic nihilism” on the part of many physicians, said Ramesh K. Ramanathan, MD, editorial board member of Clinical Cancer Research and director of the Mayo Clinic Gastrointestinal Medical Oncology program in Scottsdale, AZ, in an introduction to the latest guidelines for pancreatic cancer issued by the American Society of Clinical Oncology.
As a result, “a significant number of patients who otherwise would benefit from surgical resection are not referred to an experienced surgical team and do not receive a multimodality consultation from medical/radiation oncologists and gastroenterologists. Some patients with metastatic disease do not receive optimal systemic therapy,” while others receive inadequate symptom management or palliative care, and few participate in clinical trials, he added.2
Researchers at the Stratton VAMC in Albany, NY, observed that current National Cancer Cooperative Network (NCCN) guidelines recommend systemic therapy for metastatic pancreatic cancer (MPC) but “undertreatment of MPC continues to be an issue” nationally in a recent study published in the Journal of Clinical Oncology.3
The VA researchers sought to determine whether undertreatment also affected care of veterans with MPC. As seen nationally, the majority of veterans (52.4%) with pancreatic cancer have metastatic disease at diagnosis, said co-author Syed Mehdi, MD, lead physician for oncology and hematology at the Stratton VAMC.
The researchers analyzed treatment and survival rates for MPC using data in the VA Cancer Cube Registry on 7,585 patients treated between 2000 and 2014. They evaluated trends in treatment and compared results between hospitals that had received American College of Surgeon’s Committee on Cancer (COC) accreditation vs. those that had not.
Treatment Increased
The team found that treatment rates increased by 50% during the period studied. “We believe the increased rate of treatment is due to heightened awareness and more available treatment options. Treatment rates have increased from 27.84% in 2002 to 41.94% in 2014,” Mehdi told U.S. Medicine. Treatment rates did not differ significantly between VA hospitals with COC accreditation and non-accredited VA hospitals, 38.94% vs 38.12%, respectively. The VA treatment rates were notably lower than the 53.2% seen among COC-accredited hospitals nationally in a previous study by the same group of researchers.4
Survival rates for metastatic pancreatic cancer also improved in the VA during the study. “The use of oxaplatinin and nab-paclitaxel as part of combination drugs has increased over the years as NCCN guidelines changed. This has corresponded with an increase of 1-5 years survival of 7.84% in 2000-2004 to 14.87% in 2010-2014” in VA hospitals,” the authors wrote. They found little difference between COC-accredited hospitals and others. The one-year survival rate was 14.88%, and the five-year survival rate was 4.6%, Mehdi said, which are comparable to rates for metastatic pancreatic cancer reported nationally.
“The ‘nihilistic approach’ has evolved with better drugs and supportive care,” Mehdi noted. “New treatment combinations with FOLFIRINOX and nab-paclitaxel have emerged as the frontline combination chemotherapy for stage four pancreatic cancer. This combination is used on a case-by-case basis with good performance status in VA,” he noted.
FOLFIRINOX is a combination of folinic acid (also known as calcium folinate or leucovorin), fluorouracil (commonly called 5FU), irinotecan and oxaliplatin. Nab-paclitaxel combines paclitaxel with albumin. Other common firstline chemotherapy regimens for pancreatic cancer include gemcitabine as a monotherapy or combined with nab-paclitaxel. Pembrolizumab received FDA approval last year for pancreatic cancer with microsatellite instability.
Irinotecan liposome injection with 5FU and leucovorin received U.S. Food and Drug Administration approval in 2015 for use in patients who have progressed following treatment with gemcitabine. The FDA based its decision on a study that found that patients who received the three-drug combination lived 50% longer than those who received only 5-FU and leucovorin, 6.1 months vs. 4.2 months. The three-drug combo also doubled progression free survival compared to the dual therapy, 3.1 months vs. 1.5 months. It remains the only second-line chemotherapy for metastatic pancreatic cancer with a Category 1 recommendation by the NCCN.
“VA providers should consider referring all patients with pancreatic cancers to medical, surgical, and radiation oncology depending on the stage of cancer,” Mehdi urged. In addition, he said, “increased participation in clinical trials should be encouraged.”
A number of clinical trials are evaluating immunotherapies. A recent Phase I study of nivolumab combined with nab-paclitaxel and gemcitabine found high rates of objective response or stable disease.5
Other studies are evaluating stromal disruption using a combination of nab-paclitaxel, gemcitabine and pegvorhyaluronidase alfa (PEGPH20). A Phase II study showed that the three-drug combination with PEGPH20 improved progression-free survival by 27% and nearly doubled progression-free survival in patients with high hyaluronic acid expression.6
Clinical trials are also evaluating vitamin D analogs and high-dose vitamin C in pancreatic cancers with certain mutations.
1Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7-30. doi: 10.3322/caac.21442. Epub 2018 Jan 4.
2Ramanathan RK, ASCO 2016 Guidelines for the Treatment of Pancreatic Cancer: Why Another Guideline? ASCO Post. July 25, 2016.
3Azar I, Virk G, Esfandiaifard S, Wazir A, Khreis T, Mehdi S. Treatment and survival rates of SIV pancreatic cancer at VA hospitals: A nationwide study. J Clin Oncol. 2018 Feb 1;36(4):S507.
4Wazir A, Azar I, Mehdi S. Treatment Rates and Outcomes for Patients with Metastatic Pancreatic Cancer at a Single VA Hospital: An Exploratory Analysis. Abstract 52: 2017 AVAHO Meeting. Fed Prac. 2017 August 1.
5Wainberg ZA, Hochster HS, George B, Gutierrez M, Emery M, et al. Phase I study of nivolumab (nivo) + nab-paclitaxel (nab-P) ± gemcitabine (Gem) in solid tumors: Interim results from the pancreatic cancer (PC) cohorts. J Clin Oncol. 2017 Feb 1;35(4):S412.
6Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, et al. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018 Feb 1;36(4):359-366.


