ST. LOUIS, MO — A diagnosis of cancer of any type is terrifying. Chronic lymphocytic leukemia may be better than most, with many CLL patients able to manage the disease with regular monitoring for years or even decades. Even those who have a less-indolent form of the malignancy have a number of treatments available that can control symptoms and significantly prolong life.
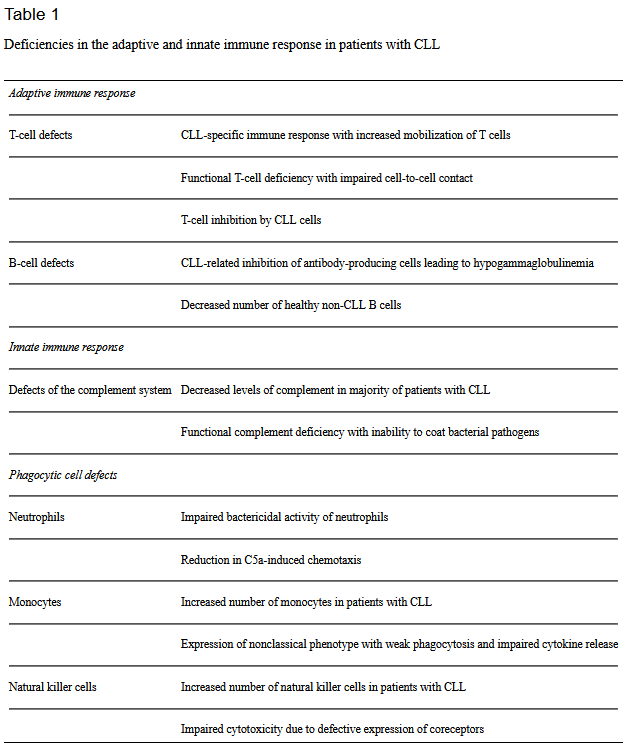
The course of the disease isn’t the only health concern for patients, however. CLL attacks white blood cells or lymphocytes, impairing the immune system. Consequently, patients with CLL are more likely to develop auto-immune diseases and infections and to develop more severe illness when they do contract an infection.
Their compromised immune system makes vaccinations especially important for this vulnerable group of patients. A VA study presented at the 2021 virtual American Society of Clinical Oncology identified a treatment that further increases the risk of a viral infection in patients treated for CLL.
Researchers led by Nirav Antao, DO, an internal medicine specialist at the St. Louis VAMC, found that herpes zoster infection was common in veterans with CLL and that receipt of chemotherapy was associated with a significant increase in the risk of infection. Herpes zoster, commonly called shingles, is a viral infection caused by the reactivation of the varicella-zoster virus, which also causes chickenpox.1
Shingles first manifests as localized, often intense, burning, tingling or numbness of the skin, followed by an itchy red rash that often forms a single line of blisters that crust over within a few days. Some people experience severe pain that can make it hard to wear clothes, sleep or bear a breeze or light touch of the skin.
Herpes zoster frequently develops around either side of the torso or along nerves in the scalp or neck. Many people also feel generally ill, with fever, chills, headache and gastrointestinal upset. The rash and pain typically resolve in a few weeks, but some individuals continue to experience symptoms for several months. After a bout of shingles, patients might develop post-herpetic neuralgia, intense nerve pain in the area affected by herpes zoster that can persist for years. Individuals who develop shingles in the area around an eye can suffer lasting damage to their vision or even blindness.
While a chickenpox vaccine has been available in the U.S. since 1996, many adults had the formerly common illness as children and remain at risk for shingles. The first vaccine specifically for shingles, Zostavax, received U.S. Food and Drug Administration (FDA) approval in 2006. Despite their increased risk of herpes zoster, Zostavax, a live attenuated virus vaccine, was not recommended for patients with CLL because it could cause shingles in immunocompromised individuals.
In 2017, a second vaccine, Shingrix, gained FDA approval. Unlike Zostavax, Shingrix does not use weakened live virus, but contains some of the components of the virus. Consequently, it cannot cause shingles. Further, Shingrix provided nearly twice the protection of Zostavax, 97% in people 50 or older vs. 51% in individuals age 60 and older. Among those over age 70, Shingrix reduced the risk of developing shingles by 97.9% compared with Zostavax’s 41% risk reduction in individuals age 70 to 79. Shingrix is recommended for individuals age 50 and older; Zostavax was pulled from the U.S. market in November 2020.
A study of patients with lymphoma, leukemia or multiple myeloma found that Shingrix was 87.2% effective against herpes zoster.2
VA Study
Despite these findings, the rate of herpes zoster (HZ) vaccination among patients with CLL remains low. The VA study demonstrates why vaccination of this vulnerable population is so important. “Infections are the leading cause of morbidity and mortality in patients with CLL,” said Antao, who presented the results at ASCO. The rash and lasting consequences from HZ infection can significantly reduce quality of life, making reducing the risk important for veterans and others with CLL.
“While the hope is herpes zoster vaccines can reduce both varicella zoster reactivation and post-herpetic neuralgia, vaccination rates remain low,” she noted.
The researchers identified 7,155 veterans who received treatment for CLL from the VA between September 1999 and October 2015. Of those, 36.9% (2,640) received first-line chemotherapy, and another 16.2% (1,161) received chemotherapy as their second line of treatment. Herpes zoster was diagnosed in 1,115 (15.5%) of the CLL patients, and 615 of the veterans received the Zostavax vaccine.
The veterans who developed shingles were slightly younger (mean 68.0 vs. 69.8 years old) and were more likely to receive treatment for CLL than those who did not develop herpes zoster (58.1% vs. 33%), although both groups had similar comorbidities.
The team found that the vaccine reduced the risk of developing herpes zoster by 29% (HR 0.71, 95% CI 0.49-1.04, p = 0.082). After adjustments for age and comorbidities, those veterans who received first-line chemotherapy had more than double the risk of shingles (HR 2.34, 95% CI 2.02-2.71, p <0.001) compared with those who did not receive chemotherapy. Chemotherapy in the second line further increased the risk of herpes zoster by 32% more than first-line therapy.
“The risk of developing HZ increases in recipients of first- and second-line chemotherapy,” the authors concluded. “In the time-varying analysis, there was a trend toward decreased infection in patients who received HZ vaccination.”
Notably, the study reviewed records from the period prior to the approval of the Shingrix vaccine. “Our study does have limitations. At the VA, we have a predominantly male population. Our study had low overall vaccination numbers and occurred during a period of time that less-effective herpes zoster vaccination was available,” Antao said.
The immunotherapy and targeted drugs commonly used today to treat CLL were not available or not widely used during the period analyzed. “The study also occurred during a time when only chemotherapeutic drugs were used for CLL,” Antao added. Because of the changes in the vaccine and in the treatment of CLL, “studies exploring infection risk in a more modern cohort, using a larger vaccinated group with the newer, more effective herpes zoster vaccine and modern chemotherapy are warranted.”
- Antao N, Chilkulwar AR, Luo S, et al. Herpes zoster in chronic lymphocytic leukemia: Effect of vaccination and treatment. Presented at: the 2021 ASCO Annual Meeting; June 4-8, 2021; virtual. Abstract 7527
- Dagnew AF, Ilhan O, Lee WS, Woszczyk D, et. Al. Zoster-039 study group. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019 Sep;19(9):988-1000. doi: 10.1016/S1473-3099(19)30163-X. Epub 2019 Aug 6. Erratum in: Lancet Infect Dis. 2020 Jan;20(1):e1. PMID: 31399377.
- Langerbeins P, Eichhorst B. Immune Dysfunction in Patients with Chronic Lymphocytic Leukemia and Challenges during COVID-19 Pandemic [published online ahead of print, 2021 Feb 25]. Acta Haematol. 2021;1-11. doi:10.1159/000514071