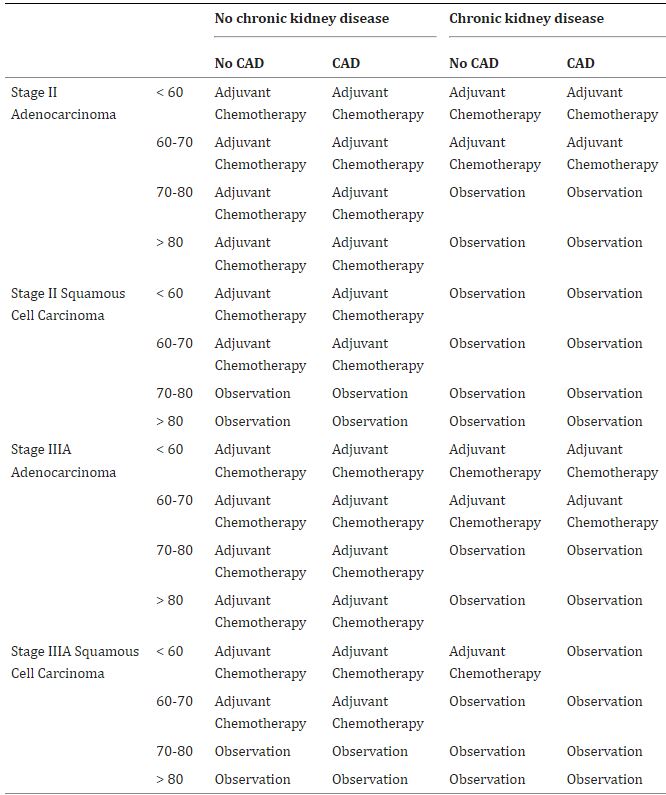
Click to Enlarge: Optimal indications for adjuvant chemotherapy for veterans without chronic obstructive pulmonary disease. Source: Current Problems in Cancer
NEW YORK — Patients with non-metastatic non-small cell lung cancer (NSCLC) face a substantial risk of recurrence and death from the disease even after potentially curative surgical resection. To reduce that risk, the use of an adjuvant platinum-based chemotherapy has been the standard of care for many years.
The risk of recurrence is far from trivial. Patients with stage I NSCLC have as much as a 25% risk of recurrence, while those with stage II have a 35% to 50% risk. Nearly 60% of stage III patients will face recurrence. The presence of undetected micrometastases, cancer cells left behind after surgery and regional involvement outside the surgical field all contribute to the high rate of recurrence.
An American Society of Clinical Oncology Guideline Rapid Recommendation Update published this year supports this practice saying “adjuvant cisplatin-based chemotherapy is recommended for all patients” with stages IIA, IIB, and IIIA NSCLC (7th edition staging system) as well as certain patients with stage IB disease. The guidelines also recommended the addition of targeted therapies in addition to chemotherapy for patients with specific mutations.1
Adjuvant chemotherapy (ACT) confers an approximately 5% improvement in five-year survival rates in clinical trials.
The evidence shows that the populations studied in clinical trials do accrue benefit from ACT. Does that apply to most patients with NSCLC? The answer to that question is much less clear.
Veterans, Too?
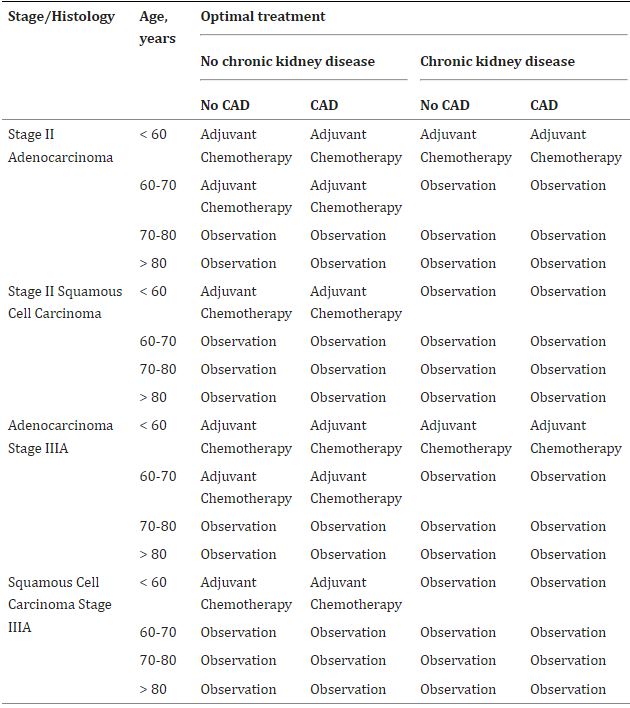
Click to Enlarge: Optimal indications for adjuvant chemotherapy for veterans with chronic obstructive pulmonary disease. Source: Current Problems in Cancer
A recent study found that ACT provides a significant benefit in both relapse-free survival and overall survival, even for patients aged 75 and older. That is noteworthy because the average age at diagnosis is about 70 years, and 38% of patients receive their diagnosis after they turn 75.2
Many of these older patients, particularly veterans, also have significant—and frequently multiple—comorbidities, unlike the majority of patients in the clinical trials. The concurrent illnesses and age contribute to longer recovery times from surgery and may reduce the benefit of ACT by delaying its initiation.
Because of the stark differences between the population studied in randomized clinical trials (RCT) and the population treated at the VA, “evidence guiding management in this population is limited and less than half of veterans with locoregional disease receive guideline-recommended therapy,” noted a team of researchers at the James J. Peters VAMC, Columbia University Irving Medical Center and the Icahn School of Medicine at Mount Sinai, all in New York.3
“Veterans with NSCLC often have major concurrent illnesses, limiting their ability to enroll in clinical trials,” they added. Among the most common comorbidities in veterans with NSCLC are chronic obstructive pulmonary disease (COPD), which affects between a quarter and half of these cancer patients, and coronary artery disease (CAD), from which up to a third suffer. Because of the high rate of diabetes among veterans, chronic kidney disease is also a common concurrent illness.
Susan Bates, MD, co-director of the James J. Peters VAMC/Columbia Cancer Center, and her colleagues at Mount Sinai sought to understand whether adjuvant chemotherapy would benefit veterans with these common comorbidities. To do so, they conducted in silico randomized controlled trials that compared adjuvant chemotherapy to observation among veterans with stage II-IIIA NSCLC.
They pulled data from previous RCTs and VA databases to create a robust simulation model based on the lung cancer policy model (LCPM), a well-validated microsimulation model of NSCLC development, progression, detection, treatment and survival in patients. Then they looked at the harms and benefits of ACT by stage, histology, age, and comorbidities.
The researchers found that for veterans who had neither COPD nor CKD, adjuvant chemotherapy provided the optimal course of action. That proved true even in patients with CAD, except those over age 70 who had squamous cell carcinoma.
Veterans who had both COPD and CKD, conversely, received a benefit from ACT only if they were less than 60 years old. Observation provided the optimal management for patients with both COPD and CKD who had squamous cell carcinoma.
For those who had CKD but not COPD, however, observation was generally the best strategy. Veterans with COPD but not CKD who were younger than age 70 and had adenocarcinoma benefited from ACT, as did those age 60 or younger with squamous cell carcinoma.
The study indicates that many veterans with common comorbidities will not benefit from ACT. “However, we also identified several groups of veterans in whom the benefits of adjuvant chemotherapy outweighed the risks of early toxicity,” the researchers concluded. Among those groups were veterans who received lobectomy for stage II-IIIA NSCLC, despite the presence of major comorbidities. “Our findings could inform patient-provider discussions and potentially reduce physicians’ uncertainty about the role of adjuvant chemotherapy in this population.”
- Pisters K, Kris MG, Gaspar LE, Ismaila N; Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA NSCLC Guideline Expert Panel. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I-IIIA Completely Resected Non-Small-Cell Lung Cancer: ASCO Guideline Rapid Recommendation Update. J Clin Oncol. 2022 Apr 1;40(10):1127-1129.
- Blasi M, Eichhorn ME, Christopoulos P, Winter H, Heußel CP, Herth FJ, El Shafie R, Kriegsmann K, Kriegsmann M, Stenzinger A, Bischoff H, Thomas M, Kuon J. Major clinical benefit from adjuvant chemotherapy for stage II-III non-small cell lung cancer patients aged 75 years or older: a propensity score-matched analysis. BMC Pulm Med. 2022 Jun 28;22(1):255.
- Bailey S, Wang Q, Kong CY, Stone K, Veluswamy R, Bates SE, Smith CB, Wisnivesky JP, Sigel K. Optimizing the use of adjuvant chemotherapy in non-small cell lung cancer patients with comorbidities. Curr Probl Cancer. 2022 Aug;46(4):100867.

