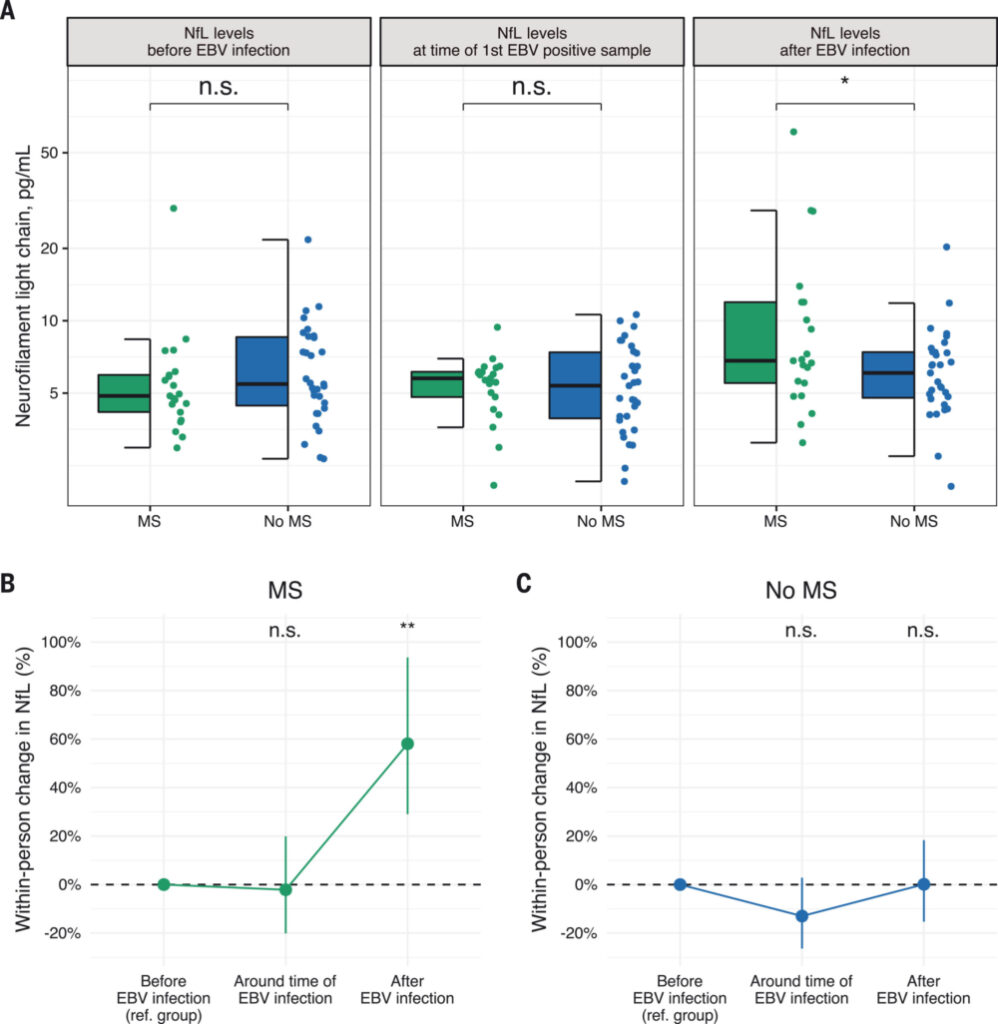
Click to Enlarge: EBV infection precedes elevation of sNfL before the onset of MS.
(A) Box plots of sNfL levels before, around, and after the time of EBV infection. *P < 0.05, two-sided multivariable linear regression model adjusted for age and sex. (B) Within-person increase in sNfL levels in MS cases around and after time of EBV infection compared with before EBV infection. **P < 0.01, two-sided linear mixed-effects regression model. (C) Within-person increase in sNfL levels in controls around and after time of EBV infection compared with before EBV infection. Error bars in (B) and (C) are 95% CIs. sNfL levels increased significantly more in MS cases than in controls in the sample collected after time of EBV infection compared with before EBV infection (P < 0.001, two-sided linear mixed-effects regression model). Source: Science
BETHESDA, MD — Researchers with the DoD provided a breakthrough in resolving the mystery surrounding the cause of multiple sclerosis (MS). Now, federal researchers at the National Institutes of Health are undertaking a clinical trial to evaluate a vaccine that could prevent MS, while another team at the Uniformed Services University of Health Sciences (USUHS) is refining a second vaccine candidate.
The massive DoD database provided the key to one of the most puzzling and persistent questions about MS—What starts the disease process? Drawing on blood samples from more than 10 million U.S. service members in the Department of Defense Serum Repository, scientists with the Uniformed Services USUHS and Harvard University were able to determine that Epstein-Barr virus (EBV) starts the cascade that eventually results in the demyelinating disease, though only in a very small percentage of infected patients.1
As an active infection, EBV causes mononucleosis. While many people have no symptoms and most others recover in weeks or months, no one fully clears the virus. Studies into the long-term effects of latent infection strongly implicate the virus in a number of other diseases, including myalgic encephalomyelitis or chronic fatigue syndrome, Hodgkin and Burkitt lymphomas, nasopharyngeal and gastric carcinomas, lupus and rheumatoid arthritis. The virus had long been suspected as the culprit in the development of MS as well, but the hypothesis proved extremely challenging to test because nearly 95% of humans contract EBV, also known as herpesvirus 4, during childhood or adolescence.
The size of the DoD database enabled the critical breakthrough. Just 5.3% of the service members with samples in the DoD serum repository had not been infected with EBV prior to the first blood sample provided as part of biennial testing. During the 20 years represented by the samples, 955 developed MS while in the service. Of those, 35 had no evidence of EBV infection at first blood collection and 34 became EBV positive prior to MS diagnosis. Of the 107 initially EBV-free participants used as a control group who did not develop MS, half became EBV positive over the period studied. Service members who remained EBV-free had virtually no chance of developing MS. On the flip side, infection with EBV increased the risk of MS more than 32 times.
“This is a big step because it suggests that most MS cases could be prevented by stopping EBV infection, and that targeting EBV could lead to the discovery of a cure for MS,” said Alberto Ascherio, professor of epidemiology and nutrition at the Harvard Chan School of Public Health and senior author of the study.
Toward a vaccine
A vaccine that prevents infection with EBV could, then, prevent MS. Given the link to a range of other diseases, it could protect health in myriad other ways as well. “A vaccine that could prevent or reduce the severity of infection with the Epstein-Barr virus could reduce the incidence of infectious mononucleosis and might also reduce the incidence of EBV-associated malignancies and autoimmune diseases,” said National Institute of Allergy and Infectious Diseases (NIAID) Director Anthony S. Fauci, MD.
NIAID, part of the National Institutes of Health (NIH), recently launched a phase I trial to evaluate an EBV vaccine. The trial is one of just two studies undertaken in more than a decade to assess such a vaccine. Principal investigator Jessica Durkee-Shock, MD, of NIAID’s Laboratory of Infectious Diseases (LID) is leading the evaluation of an EBV glycoprotein (gp)350-ferritin nanoparticle vaccine LID developed combined with a saponin-based matrix-M adjuvant developed by Gaithersburg, Md.-based Novavax Inc. Gp350 appears on the surface of EBV and on infected cells, while ferritin, which cells use to store iron, enables the protein to be displayed in a dense array on its surface.
The trial will enroll 40 healthy volunteers between the ages of 18 and 29, half of whom have had prior EBV infection and half who have no evidence of EBV infection. Participants will receive three 50 microgram injections of the vaccine, followed by a second dose 30 days later and a third 180 days after the first. The trial is expected to continue for four years.
Moderna recently launched the other phase I vaccine study in process. Its candidate, (mRNA-1189), is an mRNA vaccine that encodes EBV envelope glycoproteins gH, gL, gp42 and gp220. The four glycoproteins are instrumental in controlling entry into the B-cells and epithelial cells EBV targets. The company plans to enroll 272 participants between the ages of 18 and 30, who will receive either a placebo or one of three dosage levels on days 1, 57 and 169.
Researchers at USUHS are investigating another potential vaccine that combines recombinant EBV gH/gL with trimeric gB. The team previously demonstrated that rabbits immunized with either gH/gL or trimeric gB produced 18 to 20 times higher EBV neutralizing antibodies than gp350.
In a more recent study, the researchers showed that “the immune sera from rabbits immunized with EBV gH/gL or trimeric gB conferred strong passive immune protection of humanized mice from lethal dose EBV challenge, partially or completely prevented death respectively, and markedly decreased the EBV load in peripheral blood of humanized mice,” they wrote. “These data suggest that the combination of recombinant EBV core fusion machinery envelope proteins gH/gL and trimeric gB could be an ideal EBV prophylactic vaccine, where native epitopes could elicit high titer antibody responses both quantitatively and qualitatively.”2
- Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, Elledge SJ, Niebuhr DW, Scher AI, Munger KL, Ascherio A. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022 Jan 21;375(6578):296-301. doi: 10.1126/science.abj8222. Epub 2022 Jan 13. PMID: 35025605.
- Cui X, Cao Z, Ishikawa Y, Cui S, Imadome KI, Snapper CM. Immunization with Epstein-Barr Virus Core Fusion Machinery Envelope Proteins Elicit High Titers of Neutralizing Activities and Protect Humanized Mice from Lethal Dose EBV Challenge. Vaccines (Basel). 2021 Mar 19;9(3):285. doi: 10.3390/vaccines9030285. PMID: 33808755; PMCID: PMC8003492.

