Military Plays Large Role in Development of More-Effective Vaccine
FALLS CHURCH, VA — In response to the August 2024 upsurge of mpox in the Democratic Republic of Congo and other Africa countries, the Defense Health Agency (DHA) has produced a new flyer: Mpox—What Servicemembers SHOULD KNOW.
The upsurge in mpox, previously called monkeypox, is the second in two years to be declared a public health emergency of international concern (PHEIC) by the World Health Organization.
According to the DHA, which monitors for human-to-human transmission of mpox among DoD personnel, the risk of mpox infection to servicemembers is low and mpox is not an increased threat to servicemembers in particular.
“Though we are not at liberty to discuss specific numbers or locations of servicemembers, there have been no mpox cases among personnel in the U.S. Africa Command area of operations,” DHA officials stated in a written response to U.S. Medicine.
Mpox is a rare infectious disease caused by the mpox virus. It belongs to the same family as the virus responsible for smallpox.
Symptoms of mpox typically appear within 21 days of exposure and include a rash, which may look like pimples or blisters, on the face, mouth, hands, feet chest, or near the genitals. Other symptoms can include fever, chills, swollen lymph nodes, muscle aches, back pain, headaches, exhaustion and respiratory issues. The illness generally lasts 2 to 4 weeks.
Mpox is contagious from the onset of symptoms until all sores have healed, and new skin has formed, a process that can take several weeks. It spreads through close, personal, direct skin-to-skin contact with the rash, sores or scabs of an infected person, as well as through contact with contaminated objects, clothing, bedding, towels or surfaces. While mpox can also spread through respiratory droplets or oral fluids, it is less transmissible through airborne routes compared to respiratory illnesses such as COVID-19 or the flu.
There are two types of monkeypox virus, according to the flyer:
- Clade I, the form that prompted the current PHEIC declaration, is mainly found in Central Africa. It can make people very sick and has caused deaths at higher rates (up to 10%) in some outbreaks.
- Clade II is mainly found in West Africa and causes less severe illness. It caused the worldwide mpox outbreak in 2022. Less than 1% of people infected with clade II die.
Both clades spread the same way and have similar symptoms.
DHA officials said individuals can protect themselves by avoiding close or intimate contact with a person with mpox, practicing good hygiene and getting vaccinated if they are at high risk of exposure to the virus or have known, suspected or anticipated exposure to someone with mpox.
For those at risk of mpox infection, the Jynneos vaccine, which received Food and Drug Administration (FDA) approval for mpox in 2019, will lower that risk. Public health professionals recommend vaccination for adults who suspect they had exposure to a person with mpox, if they have certain sex-related risk factors for mpox exposure, if they think any intimate partner may have risk for mpox exposure or if they are concerned about mpox exposure in the future.
The vaccine is also recommended for travelers to certain parts of Africa if they will have difficulty avoiding mpox exposure during travel. Two doses of Jynneos vaccine, spaced 28 days apart, provide optimal vaccine protection from mpox, officials stated.
Although there is currently no treatment approved specifically for mpox, most mpox patients will recover with supportive care and pain control.
DHA officials recommend that clinicians who suspect a patient has mpox should conduct a thorough patient history, including sexual history, to assess possible exposure to mpox.
“Most patients with mpox infection have mild disease and do not require medical interventions,” they stated. “Personnel at risk for occupational exposure to mpox must adhere to appropriate workplace precautions with appropriate personal protective equipment. In addition, medical staff are offered pre-exposure vaccination commensurate with exposure risk.”
“The Department of Defense continues to monitor human-to-human transmission of mpox,” they concluded. “While it has not become a widespread threat to our forces, we are committed to the health and safety of our troops, both at home and abroad.”
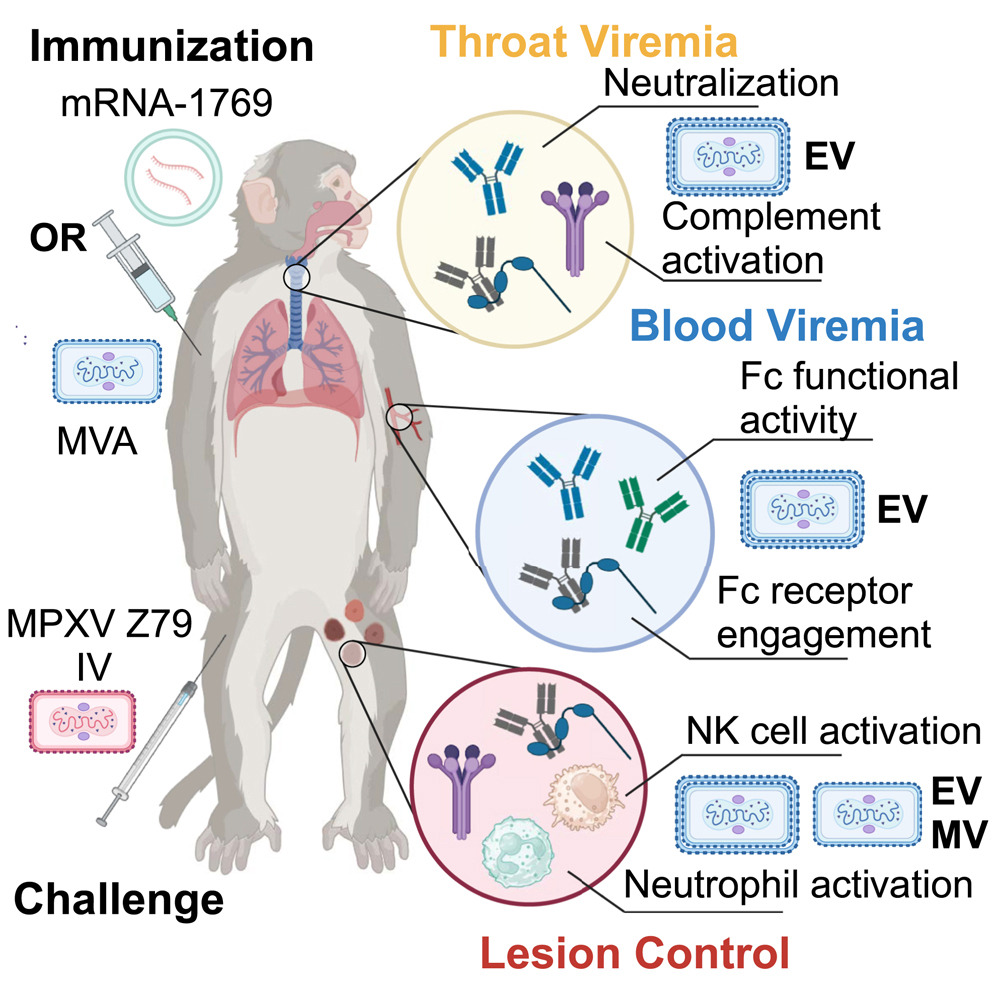
Meanwhile, research conducted by U.S. Army Medical Research Institute of Infectious Diseases scientists in Fort Detrick, MD, has led to a promising mpox vaccine.
USAMRIID’s team contributed to the design of a recent study, conducted the animal model studies and performed the immunoassay work, according to senior author Jay W. Hooper, PhD, USAMRIID’s chief of molecular virology. It was published in the journal Cell.
The current mpox vaccine is effective but has issues with cross-protection against similar viruses, incomplete immunity from mpox and adverse reactions. The new vaccine candidate, mRNA-1769, developed by Moderna, promises to address these shortcomings by using messenger RNA, or mRNA, to provide a more effective targeted approach to treating the disease, according to the article.
“With the mRNA technology, we’re able to produce a vaccine that gives quite potent responses with a very tolerable safety profile,” said Hooper said in an interview with Cell Press.
The study found that mRNA-1769 lessened the symptoms, including lesions and weight loss, and duration of the disease in animal models. The results also indicated less viral shedding in the blood and respiratory tract, which Hooper said was a highlight of the study.
“The most interesting finding was we confirmed what we had seen earlier with a DNA vaccine targeting mpox and its protection against shedding of the virus from the throat swabs, which we have not seen with the MVA-based vaccines,” he said.
“Improved orthopoxvirus vaccines are needed to strengthen our defenses against the threat posed by these viruses,” Hooper added.
Moderna’s mRNA-1769 vaccine is currently being assessed in a phase 1/2 clinical trial (NCT05995275) to address the safety and immunogenicity of an mRNA-based orthopoxvirus vaccine.
- Defense Health Agency. MPOX (formerly known as Monkeypox): What Service members SHOULD KNOW [Internet]. 2022 Aug. Available from: https://ph.health.mil/PHC%20Resource%20Library/cphe-disease-mpox-flyer.pdf
- Mucker EM, Freyn AW, Bixler SL, Cizmeci D, et. Al. Comparison of protection against mpox following mRNA or modified vaccinia Ankara vaccination in nonhuman primates. Cell. 2024 Oct 3;187(20):5540-5553.e10. doi: 10.1016/j.cell.2024.08.043. Epub 2024 Sep 4. PMID: 39236707.