Not Just for Type 1 Diabetes, CGM Now Aids Some Type 2 Patients
BALTIMORE, MD — For veterans with diabetes, managing their numbers has never been more important. While diabetes does not increase the risk of contracting COVID-19, it sharply increases the risk of severe disease and death.
Multiple studies have shown that type 1 or type 2 diabetes at least doubles and possibly quadruples the risk of death in individuals infected by SARS-CoV-2, the virus that causes COVID-19. Half of deaths in individuals younger than 65 have occurred in people with diabetes, according to the U.S. Centers for Disease Control and Prevention. Diabetics with poorly controlled serum glucose levels have the highest risk of more severe disease.
Those numbers translate into substantially greater risk for the 25% of veterans with diabetes. They also illuminate a way to improve outcomes for coronavirus infections and other infections in these veterans. It has long been known that people with diabetes have a harder time battling viral, bacterial, or fungal infections because they do not process glucose as well, they have a weaker immune response, and often have circulatory issues.
Because elevated blood sugar directly impairs immune system response, closely managing glucose levels has taken on greater importance during the pandemic for hospitalized patients with COVID-19 and people with diabetes who want to minimize their risk. As a result, the use of continuous glucose monitors (CGM) has risen markedly over the last year across all settings.
CGM Use in Hospitals
Hospitals quickly saw the advantages of CGM during the novel coronavirus outbreak. The need to reduce the risk faced by overtaxed nursing staff and preserve personal protective equipment made the pre-pandemic standard of testing for blood glucose levels untenable.
“Typically, glucose monitoring relies on point-of-care finger sticks which must happen frequently to prevent adverse events in patients with diabetes,” said Thomas J. Hornyak, MD, PhD, associate chief of staff for Research and Development at the VA Maryland Heath Care System (VAMHCS). For patients in intensive care who require insulin, standard procedures called for capillary testing of blood glucose levels every one to two hours, a requirement that became impossible to meet as hospitals converted other units, floors, and offices to intensive care units to treat COVID-19 patients, many of them diabetic. Other hospitalized patients also required frequent blood glucose testing.
Reducing or eliminating glucose monitoring wasn’t a viable option. “Studies have indicated that insulin use in hospitalized patients with diabetes predisposes them to hypoglycemia, a condition that is associated with increased morbidity and mortality. Preventing hypoglycemia requires intensive glucose monitoring,” explained Ilias Spanakis, a physician and researcher at VAMHCS and associate professor at the University of Maryland School of Medicine. Hyperglycemia has also emerged as an indicator of poor prognosis in COVID-19.
In light of the urgent need, the U.S. Food and Drug Administration issued guidance in April 2020 allowing the use of CGMs to monitor blood sugar levels in hospitalized patients during the pandemic. The American Diabetes Association (ADA), Insulin for Life, and the Diabetes Disaster Response Coalition quickly partnered with Abbott to donate 25,000 CGM sensors to hospitals across the country.
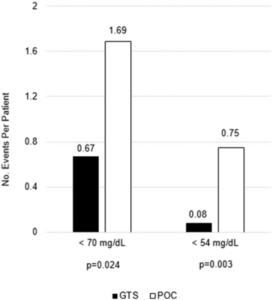
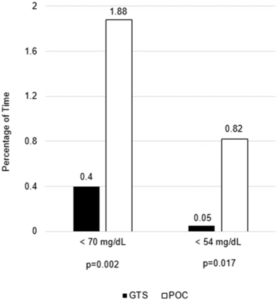
Like many hospitals around the country, VAMHCS turned to continuous glucose monitoring systems, which check glucose levels every five minutes and send the results to a centralized monitoring device at the nurses station. Spanakis and his colleagues compared CGM results to standard care in a study published in Diabetes Care.
“Dr. Spanakis’ study demonstrates that glucose levels can be monitored more frequently and efficiently,” Hornyak added. “Also, in the face of the COVID-19 pandemic, use of the continuous glucose monitoring systems proved to be prescient, showing a decrease in hypoglycemia while also safeguarding health care providers caring for virus-positive patients with diabetes.”1 Several small trials indicated CGMs offer similar benefits in ICUs and other wards.
An international panel of experts in diabetes technology, including VA researchers, issued a strong recommendation for the use of CGMs to reduce nurse contact and use of PPE for frequent glucose testing in hospitalized patients isolated with “highly contagious infectious diseases (e.g. COVID)” in September 2020.2
That recommendation followed implementation recommendations by a U.S. panel that included Spanakis and Francisco Pasquel, MD, MPH, of the Atlanta VAMC. They concluded that “CGM may be on the threshold of becoming a widely accepted form of continuous automated physiologic monitoring in the hospital setting . . . . The accommodations made in the short term enabling immediate CGM use for patients with COVID-19 create an opportunity to evaluate this technology in the inpatient setting” to obtain FDA clearance for use after the pandemic.
Outpatient use of CGM
The pandemic provided opportunities for greater use an analysis of CGMs outside the hospital, too. Restricted access to clinics and in-person appointments drove the adoption of telehealth on a broad scale, a trend likely to continue after the pandemic recedes.
“Based on the experience that we have obtained with the current crisis, I predict that several things may change in the near future, affecting drastically how we manage patients with diabetes,” Spanakis observed in an editorial. “With so many telemedicine platforms available even now, the vast majority of the outpatient visits will be transformed to telehealth appointments.”4
Because diabetes management relies on tracking numbers, the data-sharing capability of continuous glucose monitors and insulin pumps facilitates better management during virtual visits. “The abundance of data provided by CGM offers opportunities to analyze patient data more granularly than was previously possible, providing additional information to aid in achieving glycemic targets,” according to the ADA’s Standards of Medical Care in Diabetes-2021. The ADA recommends that CGM devices should be considered for all patients prescribed insulin.5
The data from CGMs help patients take more control of their diabetes between visits, too. “You can look at trend analysis and a whole bunch of different things that are derived from that technology. The data allows you to make some pretty good decisions and see what’s going on with your body,” Army veteran Jason Syr told 12WBOY. Syr’s dedicated use of a CGM allowed him to stay in the Army for six more years after his diagnosis of type 1 diabetes.
The DoD and VA have covered CGMs for type 1 diabetes for years. Just in time for the pandemic, the DoD expanded authorization to include CGM for servicemembers with uncontrolled type 2 diabetes, as well. In 2019, VA began authorizing CGMs for veterans who use insulin and require frequent blood glucose testing to manage their type 2 diabetes.
CGMs have been shown to help manage diabetes in this group. A previous study conducted at the Malcom Randall VAMC in Gainesville, Fla., found that veterans with diabetes who either required insulin or had A1c levels above 7% who received a CGM had significantly improved glycemic control and expressed 100% satisfaction with the device.6
Last November, VA relaxed its restrictions on who could use the devices to “permit COVID-19 patients to more closely monitor their glucose levels given that they are at risk for unpredictable impacts of the infection on their glucose levels and health,” said Kameron Leigh Matthews, MD.
Further, Matthews noted in a memo to VISN directors, “the use of therapeutic continuous glucose monitors may allow patients to proactively treat their diabetes and prevent the need for hospital-based diabetic care. Practitioners will also have greater flexibility to allow more of their diabetic patients to better monitor their glucose and adjust insulin doses from home by using a therapeutic continuous glucose monitor” during the pandemic.
Further expansion is likely to occur in the future. Michael Bergman, MD, section chief of endocrinology at the VA NY Harbor Healthcare System and director of NYU Langone’s Diabetes Prevention Program is studying the use of the Abbott FreeStyle Libre CGM as a screening tool for high-risk individuals in the outpatient setting. The study team also is testing the device to identify blood glucose abnormalities in veterans admitted to the Manhattan campus of the VA NYHHS with acute coronary syndrome. Picking up glucose abnormalities early, before a patient reaches prediabetic levels or has diabetes, can head off development of the disease altogether.
“So far, the data look very compelling, and if the results remain consistent, the use of the CGM would be a much simpler and practical approach to screening for glucose disorders than asking patients to undergo the [oral glucose tolerance test] in a clinical laboratory,” Bergman said. “I think it would promote more reliable screening than what is done currently.”
- Singh LG, Satyarengga M, Marcano I, Scott WH, Pinault LF, Feng Z, Sorkin JD, Umpierrez GE, Spanakis EK. Reducing Inpatient Hypoglycemia in the General Wards Using Real-time Continuous Glucose Monitoring: The Glucose Telemetry System, a Randomized Clinical Trial. Diabetes Care. 2020 Nov;43(11):2736-2743. doi: 10.2337/dc20-0840. Epub 2020 Aug 5. PMID: 32759361; PMCID: PMC7576426.
- Galindo RJ, Umpierrez GE, Rushakoff RJ, Basu A, Lohnes S, Nichols JH, Spanakis EK, Espinoza J, Palermo NE, Awadjie DG, Bak L, Buckingham B, Cook CB, Freckmann G, Heinemann L, Hovorka R, Mathioudakis N, Newman T, O’Neal DN, Rickert M, Sacks DB, Seley JJ, Wallia A, Shang T, Zhang JY, Han J, Klonoff DC. Continuous Glucose Monitors and Automated Insulin Dosing Systems in the Hospital Consensus Guideline. J Diabetes Sci Technol. 2020 Nov;14(6):1035-1064. doi: 10.1177/1932296820954163. Epub 2020 Sep 28. PMID: 32985262; PMCID: PMC7645140.
- Galindo RJ, Aleppo G, Klonoff DC, Spanakis EK, Agarwal S, Vellanki P, Olson DE, Umpierrez GE, Davis GM, Pasquel FJ. Implementation of Continuous Glucose Monitoring in the Hospital: Emergent Considerations for Remote Glucose Monitoring During the COVID-19 Pandemic. J Diabetes Sci Technol. 2020 Jul;14(4):822-832. doi: 10.1177/1932296820932903. Epub 2020 Jun 14. PMID: 32536205; PMCID: PMC7673156.
- Spanakis E. Diabetes and Technology in the Covid-19 Pandemic Crisis. J Diabetes Sci Technol. 2020 May; doi.org/10.1177/1932296820929385.
- American Diabetes Association. 7. Diabetes Technology: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021 Jan;44(Suppl 1):S85-S99. doi: 10.2337/dc21-S007. PMID: 33298418.
- Barsamyan G, Da Silva A, Whyte L, Amole M, Ghayee H, Leey JA. 855-P: Utilization of Continuous Glucose Monitoring (CGM) and Its Impact on the Care of Veterans. Diabetes. Jun 2020, 69 (S1) 855-P; DOI: 10.2337/db20-855-P.