First FDA Emergency Use Authorization Issued Last Month
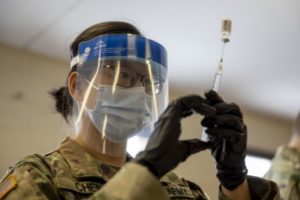
Army Spc. Ying Chen, assigned to Joint Task Force COVID-19, New York National Guard, prepares a dosage of the Pfizer-BioNTech COVID-19 vaccine at the Camp Smith Training Site Medical Readiness Clinic, NY, on Dec. 18, 2020. The New York National Guard is participating in a DoD vaccine pilot program in which 44,000 doses of the Pfizer vaccine are being administered to front line medical personnel at 16 locations around the world. Army National Guard photo by Staff Sgt. Jonathan Pietrantoni
WASHINGTON — Both the VA and DoD began distributing COVID-19 vaccine to their beneficiaries soon after the Food and Drug Administration issued an emergency use authorization for two products in mid-December.
On Dec. 11, the FDA issued the first emergency use authorization for a vaccine for the prevention of coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 16 years of age and older. That l EUA allowed the Pfizer-BioNTech COVID-19 vaccine to be distributed in the United States. Shortly thereafter, an EUA was issued for the Moderna vaccine.
VA, which said it worked in close coordination with the national Centers for Disease Control and Prevention and Operation Warp Speed, initially provided vaccinations to frontline VA healthcare workers and veterans who live in long-term care units in 37 of its medical centers across the country.
By the end of the month, the agency said it had administered initial COVID-19 vaccine doses to more than 5,000 veterans residing in its Community Living Centers and Spinal Cord Injury and Disorders Centers and more than 50,000 healthcare employees in the vaccination campaign’s first two weeks.
VA began vaccinating veterans and frontline employees at 37 initial VAMCs that had the capacity to store the Pfizer-BioNTech vaccine at extremely cold temperatures, which it requires, as well as the ability to offer high-throughput vaccination. Later, COVID-19 vaccinations were expanded to another 128 sites using the two products, both of which require two doses for maximum efficacy.
“I couldn’t be happier with the work VA employees are doing in taking this important first step toward the end of the pandemic,” said VA Secretary Robert Wilkie. “Successfully implementing a plan like this in the nation’s largest healthcare system takes planning, collaboration and teamwork. As vaccines become more widely available, we will continue to implement our plan to offer them to any veteran or employee who wants one at no cost.”
Ultimately, VA plans to offer COVID-19 vaccinations to all veterans and employees who want to be vaccinated when supplies increase.
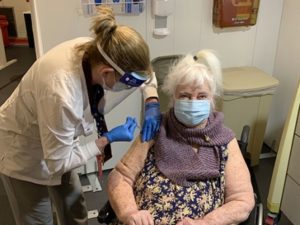
RN Sandra Getchell gave the COVID-19 vaccine to World War II
veteran Margaret Klessens, aged 96, a resident of VA Bedford Healthcare System’s community living center on Dec. 14. She was the first VA patient
nationwide to receive the COVID-19 vaccine. VA photo by Kat Bailey
The agency said it decided to prioritize vaccines for healthcare workers because they are at high risk for contracting and spreading COVID-19 to other staff members and patients and that they need to stay healthy to ensure the continued care of veterans who depend on them.
Meanwhile, the Defense Health Agency director, Lt. Gen. Ronald Place, said the DoD also moved quickly after the EUA was issued. “Thirteen military communities in the United States received their vaccine doses on time and within 24 hours of receipt, we began administering the vaccine according to plan,” Place said.
DoD mobilized to distribute approximately 44,000 initial doses to those facilities, the first step in a plan to prioritize, distribute and vaccinate military personnel.
“As the vaccine becomes more widely available in the coming weeks, we strongly encourage everyone to get vaccinated,” Place emphasized.Place said the DHA is gathering real-time data from the pilot sites to fine-tune any process issues in advance of larger scale distribution. “We are intensely focused on the feedback we are getting because these initial sites form the foundation to validate our distribution, administration, and reporting processes, which will then inform how we expand to additional distribution sites,” he said.
At Tripler Army Medical Center, for example, prioritized DoD personnel at each location were in line to receive the vaccine as soon as shipments arrived.
Navy Medical Center San Diego also received its initial shipment Dec. 14 and started vaccinating the following day. Immunized were healthcare workers within the emergency department and intensive care unit, as well as local installation emergency medical services, fire and police departments from six local Navy and Marine Corps installations it serves.In choosing the initial sites, DoD leadership looked at the size of the DOD population of high priority personnel, cold storage capability and sufficient medical personnel to administer the vaccines and monitor recipients after receiving them.
Priority Groups
At the VA, where LTC residents will be the first patient group to be vaccinated, factors such as age, existing health problems and other considerations that increase the risk of severe illness or death from COVID-19 will be used to designate the next priority group.
“VA is well prepared and positioned to begin COVID-19 vaccinations,” said Wilkie. “Our ultimate goal is to offer it to all veterans and employees who want to be vaccinated.”
The VA sites chosen for initial distribution of the vaccine will closely monitor patients and staff for side effects and log this information in its vaccine monitoring and tracking system. That system already is place for vaccinations such as influenza and shingles.
VA will report directly to the CDC data on all vaccine doses administered by VA. The department also will provide general, public updates on the number of people being vaccinated at the sites, similar to how COVID-19 testing figures are posted.
The DoD advised that, as lessons from the control pilot are analyzed and more vaccines are made available over time, additional locations will be added, and more personnel will become eligible for vaccinations. Eventually, said Assistant Secretary of Defense for Health Affairs Thomas McCaffery in a press conference shortly after the EUA was issued, that will extend to all DoD personnel, including active and selective reserve components, including the National Guard, family members, retirees, DOD civilians and even some DoD contractors.
“Future allocations of the COVID-19 vaccine will focus on vaccinating priority populations quickly and safely, while simultaneously refining the intricate planning for the delivery of larger volumes of vaccine in future waves,” McCaffery said.
At the same press conference. Place encouraged DoD personnel to take the vaccine when it becomes available. Unlike many immunizations, the COVID-19 vaccine is voluntary, even for active-duty military personnel.
“As with most vaccines, some people may experience small adverse effects such as arm soreness, fatigue, even a fever,” Place added. “The department will be fully transparent about any adverse effects that are reported and will share this information with the CDC.”
The DoD also is prepared to help vaccinate civilians. The U.S. Department of Health and Human Services now is allowing states to use National Guard health and medical personnel to increase access to COVID-19 vaccinations.

