Drug Used as Preexposure Prophylaxis in High-Risk Groups
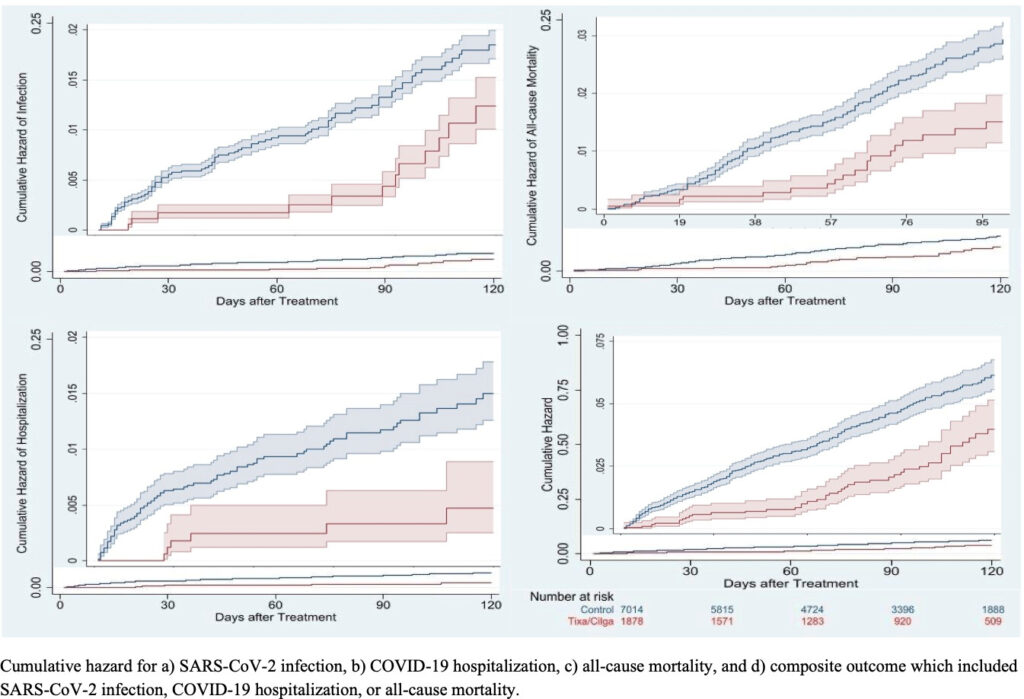
Click to Enlarge: Cumulative risk of COVID-19 outcomes for tixagevimab/cilgavimab recipients compared to untreated controls. Source: American Society for Microbiology
HINES, IL — The risk of COVID-19 morbidity and mortality is much higher for immunocompromised patients, and, while vaccines have been highly successful at preventing the spread of SARS-CoV-2 and decreasing the likelihood of severe disease in the general population, those who are immunocompromised still face a greater danger for breakthrough infections and persistent viral replication, according to a new study.
The report in mBio noted that the PROVENT study, a phase 3, multicenter, randomized, and placebo-controlled trial, demonstrated that a single dose of intramuscular tixagevimab/cilgavimab, marketed as Evusheld, significantly reduced the incidence of symptomatic SARS-CoV-2 infection by 76.7% after 90 days in a varied group of adults with an increased risk of inadequate response to vaccination and/or increased risk of exposure to SARS-CoV-2.1
Based on these findings, the U.S. Food and Drug Administration (FDA) granted in December 2021 an emergency use authorization (EUA) of tixagevimab/cilgavimab as pre-exposure prophylaxis for moderate-to-severe immune compromised individuals or for whom vaccination with any available COVID-19 vaccine is not recommended due to a history of severe adverse reaction.
While the PROVENT trial also included those with chronic health conditions that could predispose individuals to COVID-19 complications, according to VA researchers, only 11% of the trial participants were immunocompromised, defined as those on immunosuppressive therapy or having an immunosuppressive disease or cancer. They explained that “treatment effectiveness in this crucial subgroup could not be estimated in the trial. Furthermore, all participants in the PROVENT trial were unvaccinated at the time of trial entry; therefore, indications for tixagevimab/cilgavimab among vaccinated persons remain unknown.” Because the follow-up of participants in the PROVENT trial ended in September 2021, the authors suggested that “an analysis regarding “real-world” effectiveness is needed for tixagevimab/cilgavimab among vaccinated immunocompromised veterans after the emergence of the Omicron variant (December 2021 in the United States).”
The retrospective cohort study led by the VA’s PBM, Center for Medication Safety in Hines, IL, and including VA researchers from the Atlanta VAMC; the VA Office of Research and Development in Washington, DC; the White River Junction, VT, VAMC; and the VA Northeast Ohio Healthcare System in Cleveland, included immunocompromised veterans age as of Jan. 1, 2022, who were receiving VA healthcare. The study team compared a cohort of 1,878 patients treated with at least one dose of intramuscular tixagevimab/cilgavimab to 7,014 matched controls selected from patients who met study criteria but were not treated.
With patients followed through June 15, 2022, or until death, whichever occurred earlier. The primary outcome was defined as a composite of SARS-CoV-2 infection, COVID-19-related hospitalization and all-cause mortality.
Most, 73%, of the tixagevimab/cilgavimab recipients were 65 and older, with 80% having had three or more mRNA vaccine doses or two doses of Ad26.COV2.
Lower Rates of SARS-CoV-2
“Compared to matched controls, recipients had a lower incidence of the composite COVID-19 outcome (49/1,878 [2.6%] vs. 312/7,014 [4.4%]; HR 0.35; 95% CI, 0.24-0.52), and individually SARS-CoV-2 infection (HR 0.44; 95% CI, 0.22-0.88), COVID-19 hospitalization (HR 0.24; 95% CI, 0.10-0.59), and all-cause mortality (HR 0.32; 95% CI, 0.19-0.55),” the researchers pointed out, adding, “In conclusion, tixagevimab/cilgavimab was associated with lower rates of SARS-CoV-2 infection and severe COVID-19 during the Omicron BA.1, BA.2, and BA.2.12.1 surge.”
-Background information in the report advised that fast-tracking medications to help mitigate the COVID-19 pandemic meant that clinical trials have been limited. That “created the need to a real-world analysis, using electronic health record data, of the effectiveness of tixagevimab/cilgavimab for the prevention of COVID-19 infection in the unique population of U.S. veterans. Unlike those in the PROVENT clinical trial from which the emergency use authorization for tixagevimab/cilgavimab as a preventative treatment arose, the veteran population is highly immunocompromised and nearly 96% totally vaccinated.”
The authors explained that those demographics allowed analysis of the effectiveness of tixagevimab/cilgavimab in preventing COVID-19 under different conditions in a population that was more fragile than the one used for the initial clinical trial.
“In this retrospective cohort study using data from veterans across the VHA, the largest integrated healthcare system in the United States, administration of tixagevimab/cilgavimab was associated with a significant reduction in the risk of SARS-CoV-2 infection, COVID-19 hospitalization, and all-cause mortality among recipients, compared with untreated, matched controls,” the researchers concluded. “These findings were observed among severely immunocompromised veterans, further supporting the EUA criteria for the use of tixagevimab/cilgavimab in this population. In addition, evidence of augmented protection against SARS-CoV-2 infections among vaccinated immunocompromised veterans who received tixagevimab/cilgavimab was found, akin to that of the population of fully boosted adults who were not immunocompromised.”
The study team pointed out that the EUA encourages the use of tixagevimab/cilgavimab primarily among fully vaccinated immunocompromised patients; “however, none of the participants in the PROVENT trial were vaccinated. In comparison, nearly all (96%) of our study population received at least two doses of a COVID-19 mRNA vaccine or one dose of Ad26.COV2 before receiving tixagevimab/cilgavimab. In PROVENT, only 11% of trial participants were immunocompromised, compared to all veterans in the current analysis. Furthermore, patients aged 65 years and older accounted for a small proportion (24%) of patients included in the PROVENT trial, compared to 73% of those in the current study.”
On the other hand, they emphasized that only a small proportion of eligible veterans received treatment, calling for enhanced education and outreach so that more immunocompromised veterans treated by the VHA receive the medication, specifically during COVID-19 surges.
- Young-Xu Y, Epstein L, Marconi VC, Davey V, et. al. Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: retrospective analysis of National Veterans Health Administration electronic data. mBio. 2023 Aug 3:e0102423. doi: 10.1128/mbio.01024-23. Epub ahead of print. PMID: 37535398.

