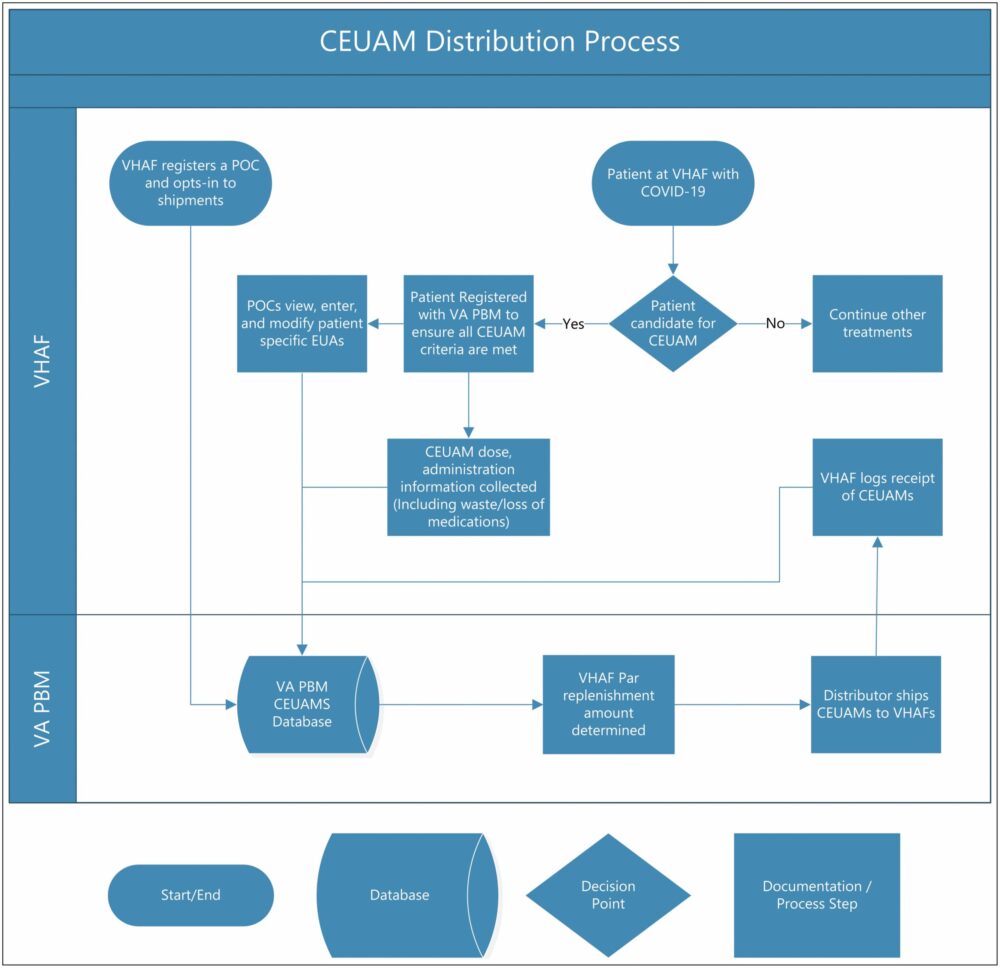
Click to Enlarge: Process diagram. CEAUM indicates COVID-19 emergency use–authorized medication; COVID-19, coronavirus disease 2019; EUA, emergency use authorization; POC, point of contact; VAMC, Veterans Affairs Medical Center; VA PBM, Veterans Affairs Pharmacy Benefits Management. Source: American Journal of Health-System Pharmacy
WASHINGTON, DC — Treatments used under emergency-use authorizations (EUA) have led to significant reductions in morbidity and mortality during the COVID-19 pandemic, but ensuring their safe use in the people who could stand to benefit the most from them is a formidable task—particularly for the nation’s largest health system.
As of Sept. 22, 2022, 18 such drugs and four vaccines had been used under EUAs—which authorize products for use in limited situations, such as public health emergencies, and are issued with requirements that healthcare facilities must meet by collecting and reporting data.
When the first COVID-19 emergency use-authorized medication (CEUAM), remdesivir, was approved, it immediately became necessary for the VHA to describe the requirements for EUA, to distribute the medication—and any subsequent ones, as more EUAs were granted—in an equitable manner and to ensure compliance with all EUA requirements. To determine the most-efficient way to accomplish this, VHA Pharmacy Benefits Management Services, (PBM) coordinated an interdisciplinary team including clinical providers, pharmacy personnel and ethics experts.
Given the number of facilities in the VHA—170 VAMCs and 1,200 outpatient clinics—and their assortment of sizes and complexity of resources, the team determined that a centralized EUA distribution process would conserve resources and ensure distribution, said Paul Fina, PharmD, a clinical pharmacist at the VA Center for Medication Safety (VA MedSAFE). Such a system would avoid the need for each facility to develop its own processes and distribution, as well as the need for facility-specific personnel to manage the processes. It also would provide standardization toward meeting all EUA requirements, he said.
On that recommendation, VHA PBM developed a centralized process, a SharePoint website and a dashboard to collect, aggregate and track necessary data and ensure compliance in a national integrated healthcare system.
Fina and colleagues at VA MedSAFE, VHA PBM and VA Center for Health Equity Research and Promotion described the process, the dashboard and their results in the Dec. 25, 2022, issue of the American Journal of Health-System Pharmacy.1
“The centralized distribution process facilitated medication delivery to facilities, ensured ample inventory, and promoted medication tracking, which helped to ensure fair and equitable distribution of CEUAMs across VHA,” the authors wrote.
Transparency in Distribution
“The dashboard was a comprehensive location for healthcare providers, local leaders, and national leaders to obtain and view necessary information about CEUAMs,” the authors wrote. “The dashboard provided transparency into the ordering, distribution, and administration processes for the EUA medications.”
Dashboards were refreshed daily and could be viewed in aggregate or at the individual patient level, VAMC level, or Veterans Integrated Services Network level (an area incorporating multiple VAMCs in close proximity in neighboring states), according to the authors. The dashboard also could be filtered to view certain products and/or timeframes, and each page provided to offer different medication safety information to the user was improved with a passive surveillance page, which included summarized individual patient characteristics along with safety data reported to the VA ADE Reporting System (ADERS) and FDA MedWatch, allowing for quick identification of the most commonly reported adverse events.
While the development of the dashboard was somewhat resource-intensive for VHA BPM—approximately 80 hours initially and another 10 to 20 hours weekly to maintain and develop further—the time savings from that investment have been dramatic.
“The time savings with the dashboard are estimated to be, at a minimum, 1 to 2 full-time-equivalent employees at approximately 200 facilities, as each site receiving CEUAMs would need to implement a process to order the medications, track distribution and administration, and audit the prescribing and distribution process to ensure compliance with CEUAM requirements. In addition, each facility would need to independently upload the required information on CEUAMs to the Centers for Disease Control and Prevention and Department of Health and Human Services. “At a time when all healthcare resources are stretched, the accumulated time savings are invaluable.”
Although the dashboard was developed specifically for COVID-19 medications and vaccines, Fina said it could be used for other drugs or treatments. In fact, recently, it has been used to manage tracking monkeypox vaccines and therapeutics under similar centralized allocation by the federal government.
And while it was developed specifically for and used exclusively by the VHA “other systems may use it as a model to develop their own, which was our hope in publishing the manuscript,” he said.
- Shumunov A, Fina P, Martin J, Barrett A, Echevarria K, Cunningham F. Emergency use authorization medication distribution, utilization, and monitoring in a national integrated healthcare system. Am J Health Syst Pharm. 2022 Dec 25:zxac386. doi: 10.1093/ajhp/zxac386. Epub ahead of print. PMID: 36566503.

