Recent Satisfaction, Safety Data Available for Ruxolitinib Cream
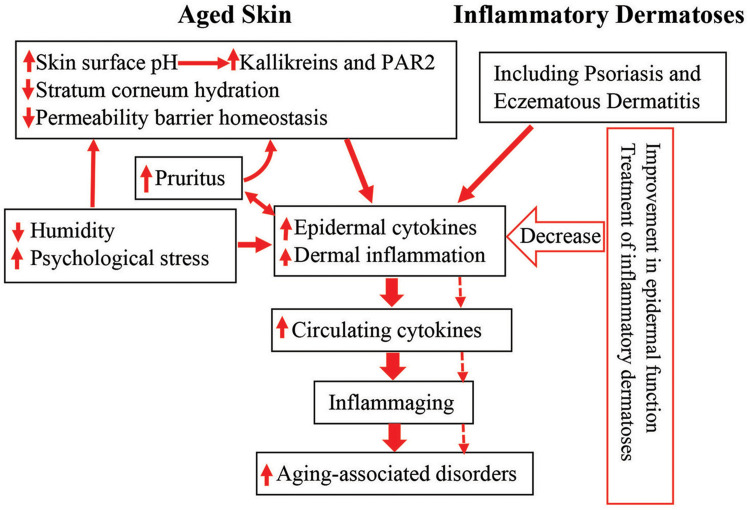
Click to Enlarge: Schematic diagram: link between cutaneous conditions and aging-associated disorders in the elderly. Aged humans exhibit epidermal dysfunction. Both epidermal dysfunction and inflammatory dermatoses can provoke cutaneous inflammation. Prolonged cutaneous inflammation can result in inflammaging, leading to the development of inflammaging-associated disorders in the elderly (indicated in solid arrows). Conversely, either appropriate treatment of inflammatory dermatoses or improvement in epidermal function can decrease cutaneous inflammation, preventing the development and progression of inflammaging, consequently alleviating inflammaging-associated disorders in the elderly (indicated in dotted arrows).
Abbreviation: PAR2, protease-activated receptor 2. Source: Dove Press – Clinical Interventions In Aging
SAN FRANCISCO — Aging can lead to the development of cutaneous symptoms and disorders, and in many older adults the effects often are not limited to the skin, according to a new VA study.
“Although the pathogenesis of these aging-associated disorders is not clear yet, abnormalities in the skin are linked to some aging-associated disorders at least to some extent,” wrote researchers from the San Francisco VAMC and the University of California, San Francisco with Chinese colleagues. “Inflammatory dermatoses such as psoriasis and atopic dermatitis predispose to the development of cardiovascular diseases, obesity and type 2 diabetes.”
Their report in Clinical Interventions in Aging also pointed out that both chronologically aged skin and patients with some aging-associated systemic conditions display altered epidermal function. Those included reduced stratum corneum hydration levels, which can provoke cutaneous inflammation.1
“Because aged skin exhibits higher expression levels of inflammatory cytokines, which play a pathogenic role in a variety of aging-associated health condition, the association of the skin with some aging-associated disorders is likely mediated by inflammation,” the authors suggested. “This postulation is supported by the evidence that improvement in either epidermal function or inflammatory dermatoses can mitigate some aging-associated disorders such as mild cognitive impairment and insulin sensitivity.”
Whatever the cause, the findings hold special significance for VA healthcare, where the average patient is 58 years old. In fact, 37.1% of VHA patients are 65 and older, with another 41.2% between 45 and 64.
“Although the pathogenesis of aging-associated disorders is not fully elucidated yet, the crucial role of chronic, low-grade inflammation, often termed ‘inflammaging,’ in the development of these chronic disorders is hypothesized,” according to the study team. “Indeed, aged humans display higher circulating levels of proinflammatory cytokines and C-reactive protein. Inflammation has been linked to the pathogenesis of aging-associated diseases, such as type 2 diabetes, obesity, and Alzheimer’s disease. Correspondingly, inflammatory dermatoses are the risk factors of aging-associated health conditions”.
The article pointed out that atopic dermatitis is among the common inflammatory dermatosis linked to aging-associated disorders such as obesity and mild cognitive impairment, with the association of atopic dermatitis with central obesity being stronger in females than in males.
Atopic dermatitis also is a risk factor for other aging-associated disorders, the researchers advised. They cited studies indicating that AD increases the risk for multiple cardiovascular events, including myocardial infarction, stroke, heart failure, ischemic stroke and angina.
Specifically, the study recounted researchers showing that the prevalence of cardiovascular diseases is more than 1% higher in individuals with atopic dermatitis than in the controls (5.5% vs. 4.1%), with a hazard ratio of 1.42 (95% CI 1.33-1.52). Atopic dermatitis also increases the risk for hyperlipidemia and hypertension with hazard ratio of 33.02 (95% CI 32.37–33.69, p < 0.0001) and 4.86 (95% CI 4.65-5.09, p < 0.0001), respectively. It also is associated with coronary heart diseases with an adjusted odds ratio of 1.96 (95% CI 1.02-3.77, p = 0.04), although one study suggested that AD decreases the risk for myocardial infarction and hypertension.
While the association between atopic dermatitis and diabetes mellitus is inconclusive, Type 2 diabetes patients are much more likely to have atopic dermatitis, the study reported.
“Recent studies demonstrated that atopic dermatitis also increases the risk of dementia and Alzheimer’s diseases, with hazard ratio of as high as 3.74,” the authors wrote. “The link between atopic dermatitis and aging-associated disorders is also evidenced by a positive correlation of the prevalence of these disorders with the severity of atopic dermatitis. Hence, atopic dermatitis is associated with some aging-associated disorders.”
How does this information affect treatment decisions about atopic dermatitis in older patients, especially with some studies suggesting the polypharmacy rate could be as high as 79%?
Some recent studies suggested topical agents as a possibility. For example, a report this spring in Dermatology & Therapy found effective disease control and physician satisfaction with ruxolitinib cream for the treatment of AD in adults in a clinical practice setting.
Outcomes were similar whether ruxolitinib cream, a topical formulation of ruxolitinib, a potent, selective inhibitor of Janus kinase (JAK) 1 and JAK2, was prescribed as monotherapy or in combination regimens.2
The University of San California, San Diego-led study said those findings suggest “a role for ruxolitinib cream across the spectrum of disease.”
The 2021 U.S. approval of ruxolitinib cream for treatment of AD in patients 12 and older with mild to moderate disease was based on the results of two pivotal phase 3 studies. Until this study, real-world data to describe the effectiveness of ruxolitinib cream and physician satisfaction with treatment had been limited.
The study team sought to elucidate disease control among adults with mild to moderate AD prescribed ruxolitinib cream, as well as physician satisfaction with treatment.
For the study, data were analyzed from the Adelphi AD Disease Specific Programme, a U.S. real-world, cross-sectional survey of physician-reported data, undertaken between August 2022 and March 2023. For adult patients, physicians reported patient demographics, clinical characteristics, treatment patterns and physician satisfaction with disease control.
Of 1,360 patients with AD in the physician-reported survey, 149 patients had received ruxolitinib cream (in combination or as monotherapy) for a month or more, including 59 patients receiving monotherapy. Prior to treatment with ruxolitinib cream, 84.6% of patients had moderate AD (Investigator’s Global Assessment, IGA of 3), but, after treatment (median duration, 26 weeks), only 21.5% had an IGA of 3, with 48.3% of patients having clear or almost clear skin (IGA of 0/1).
For those patients, 81.2% were not currently experiencing a flare, and physicians were satisfied with disease control for 87.3%, according to the authors, who pointed out that results were similar in patients receiving monotherapy. They said the most frequent physician-reported reasons for prescribing ruxolitinib cream included relieving itch, improving lesion redness/thickness, achieving disease control and reducing/controlling flares.
Also, this spring, a study in the American Journal of Clinical Dermatology reported on post-marketing safety data finding that ruxolitinib cream is generally well tolerated, without significant systemic adverse effects and with a low incidence of application site reactions.3
Ruxolitinib cream was the first topical Janus kinase (JAK) inhibitor approved in the United States for the treatment of mild to moderate atopic dermatitis and nonsegmental vitiligo. “Because ruxolitinib cream is indicated for inflammatory conditions, it is subject to the same warnings as oral JAK inhibitors in the U.S.,” the report noted.
A post-marketing study with oral tofacitinib, approved for rheumatoid arthritis, had triggered class warnings for JAK inhibitors, including the risk of serious infections, mortality, malignancy, major adverse cardiovascular events and thrombosis. In response, nearly 14,000 patient-years of post-marketing safety data from the first year following market approval of ruxolitinib cream were reviewed.
To accomplish that, researchers queried the Incyte global safety database from Sept. 21, 2021, to Sept. 20, 2022, and the U.S. Food and Drug Administration Adverse Event Reporting System as of Sept. 30, 2022, for adverse event (AE) reports received for ruxolitinib cream.
That search identified 294 post-marketing individual case safety reports containing 589 events, including four serious AEs and no fatal AEs. AEs, defined as any unfavorable sign, symptom or disease, representing more than 2% of all events included application site pain (n = 16), atopic dermatitis (n = 15), skin irritation (n = 15), scratch (n = 14) and condition aggravated (n = 13). The four serious AEs were skin cancer (n = 2), pericarditis and thrombocytopenia (both n = 1), none of which had sufficient information to assess possible relatedness to ruxolitinib cream.
None of the serious AEs associated with the class warnings for JAK inhibitors were reported.
- Yang B, Man MQ. Improvement in Cutaneous Conditions Can Benefit Some Health Conditions in the Elderly. Clin Interv Aging. 2023 Dec 1;18:2031-2040. doi: 10.2147/CIA.S430552. PMID: 38058550; PMCID: PMC10697145.
- Eichenfield LF, Liu J, Marwaha S, Piercy J, Sturm D, Anderson P. Satisfaction with Control of Mild to Moderate Atopic Dermatitis with Ruxolitinib Cream: US Physician and Patient Perspectives. Dermatol Ther (Heidelb). 2024 Mar;14(3):685-696. doi: 10.1007/s13555-024-01116-0. Epub 2024 Mar 8. PMID: 38453811; PMCID: PMC10965874.
- Hu W, Thornton M, Livingston RA. Real-World Use of Ruxolitinib Cream: Safety Analysis at 1 Year. Am J Clin Dermatol. 2024 Mar;25(2):327-332. doi: 10.1007/s40257-023-00840-1. Epub 2024 Jan 19. PMID: 38243107; PMCID: PMC10866801. Dermatol Ther (Heidelb)

