Click to Enlarge: CV indicates cardiovascular; NNH, number needed to harm; NNT, number needed to treat. Source: JAMA Network Open
PALO ALTO, CA — The Systolic Blood Pressure Intervention Trial (SPRINT) found benefits of intensive vs. standard blood pressure (BP) control. But do those findings generalize to adults with chronic kidney disease (CKD)?
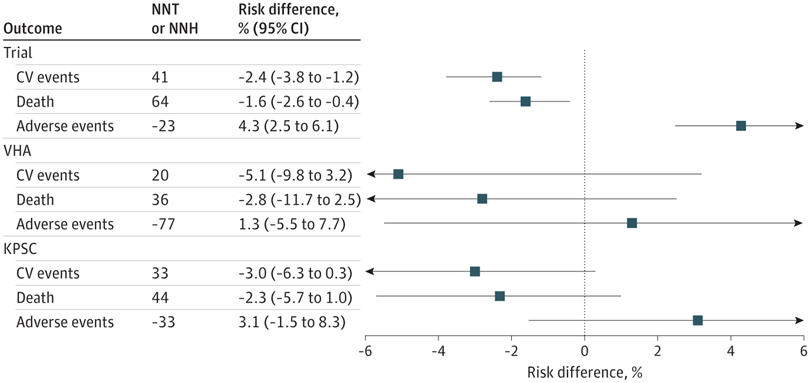
That question was answered in a recent study led by Stanford University School of Medicine and the VA Palo Alto, CA. Their comparative effectiveness study of 99,921 SPRINT-eligible adults with CKD from two healthcare systems—one of them the VHA—used transportability analysis to estimate mean treatment effects. Results indicated that intensive vs. standard BP control was associated with comparable relative benefits and even larger absolute benefits than those observed in SPRINT.
“These results suggest potential population-level benefits of intensive BP targets in SPRINT-eligible adults with CKD,” the researchers wrote in JAMA Network Open.1
The study team pointed this out: “It is unclear whether the effects of intensive vs. standard blood pressure (BP) targets seen in clinical trials generalize to patients with chronic kidney disease (CKD) encountered in everyday practice due to differences in the distribution of cardiovascular risk factors and coexisting conditions.”
The comparative effectiveness study focused on populations with CKD at the VHA and enrolled in Kaiser Permanente of Southern California (KPSC). All participants met the eligibility criteria for SPRINT between Jan. 1 and Dec.31, 2019. Major cardiovascular events, all-cause death, cognitive impairment, CKD progression and adverse events at 4 years were defined as the main outcomes, with analysis was performed between May 2023 and October 2024.
Ultimately, 85,938 patients (mean [SD] age, 75.7 [10.0] years; 81 628 [95.0%] male) from the VHA and 13 983 patients (mean [SD] age, 77.4 [9.6] years; 5371 [38.4%] male) from KPSC were included. Compared with 9,361 SPRINT participants (mean [SD] age, 67.9 [9.4] years; 6029 [64.4%] male), the patients were older, had less prevalent cardiovascular disease, higher albuminuria and used more statins.
“The associations of intensive vs standard BP control with major cardiovascular events, all-cause death, and adverse events were transportable from the trial to the VHA and KPSC populations; however, the trial’s effects on cognitive and CKD outcomes were not transportable in one or both clinical populations,” the authors explained.
They found that intensive vs. standard BP treatment was associated with lower absolute risks for major cardiovascular events at 4 years by 5.1% (95% CI, -9.8% to 3.2%) in the VHA population and 3.0% (95% CI, -6.3% to 0.3%) in the KPSC population and higher risks for adverse events by 1.3% (95% CI, -5.5% to 7.7%) in the VHA population and 3.1% (95% CI, -1.5% to 8.3%) in the KPSC population.
“In this comparative effectiveness study, the reduction in fatal and nonfatal cardiovascular end points and the increase in adverse events observed in SPRINT were largely transportable to trial-eligible CKD populations from clinical practice, suggesting benefits of implementing intensive BP targets,” the researchers pointed out.
Hypertension Mortality
Management of CKI seeks to reduce morbidity attributable to hypertension, with guidelines from the Kidney Disease Improving Global Outcome (KDIGO) recommending treatment of hypertension to a target systolic blood pressure (BP) of less than 120 mm Hg when tolerated. That recommendation is based on SPRINT and its prespecified subgroup analyses in participants with CKD. SPRINT found that treatment to a systolic BP less than 120 mm Hg vs. less than 140 mm Hg reduced mortality, cardiovascular events and mild cognitive impairment; increased certain adverse events, such as acute kidney injury, while having no effect on CKD progression.2
“One of the main points of controversy concerning the KDIGO BP target is the uncertain generalizability of SPRINT to adults with CKD in clinical practice,” the authors advised. “Studies generalizing SPRINT treatment to the U.S. population using NHANES data are limited by the small number of individuals with advanced CKD in the NHANES sample. Furthermore, although randomized trials have strong internal validity, they may not reflect the risks and benefits of treatment in clinical populations. Trial eligibility criteria and enrollment procedures result in study populations with different clinical characteristics than patients in routine practice.”
The authors noted that their study complemented meta-analyses and the SPRINT CKD subgroup analysis in three important aspects. First, we found that the trial and clinical populations differed in several characteristics, including age, sex, prior cardiovascular disease, smoking history, and statin use, and the clinical populations were also markedly different from each other. Nevertheless, the mean treatment effects on the primary outcomes in both clinical populations were consistent with those observed in the trial. This observation suggests that most baseline covariates in the models are not effect modifiers for the outcomes on the relative scale, supporting the rationale for BP targets based on absolute risk rather than clinical characteristics.
“Second, our analysis evaluates multiple secondary outcomes and adverse events. By including a broader set of outcomes, our analysis assesses benefits relative to the risk of harm. Third, this analysis provides insights into the generalizability of BP targets to patients with advanced CKD, a subgroup with limited representation in SPRINT. By demonstrating that intensive BP control had similar associations with the primary cardiovascular and mortality endpoints but higher risks for certain adverse events in this subgroup, these findings may help to reconcile conflicting or inconclusive results from previous trials and observational studies of BP control.”
The study added, “For similar estimated relative risk reductions between the trial and clinical CKD target populations, there were larger projected absolute benefits in the CKD target populations and similar or smaller absolute harms.”
The researchers explained that that meant a more favorable benefit-to-harm ratio in the VHA and KPSC CKD populations compared with the trial population. “Using the VHA CKD population as an example, for every 4 patients who have a cardiovascular event prevented with intensive BP treatment, 1 patient would experience a serious adverse event. In the KPSC CKD population, for every 1 patient who has a cardiovascular event prevented with intensive BP treatment, 1 would experience a serious adverse event. In contrast, in the trial population, for every 3 patients who have a cardiovascular event prevented, 5 would experience a serious adverse event.”
- Kurella Tamura M, Huang M, An J, et al. SPRINT Treatment Among Adults With Chronic Kidney Disease From 2 Large Health Care Systems. JAMA Netw Open. 2025;8(1):e2453458. doi:10.1001/jamanetworkopen.2024.53458
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, et. Al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015 Nov 26;373(22):2103-16. doi: 10.1056/NEJMoa1511939. Epub 2015 Nov 9. Erratum in: N Engl J Med. 2017 Dec 21;377(25):2506. doi: 10.1056/NEJMx170008. PMID: 26551272; PMCID: PMC4689591.

