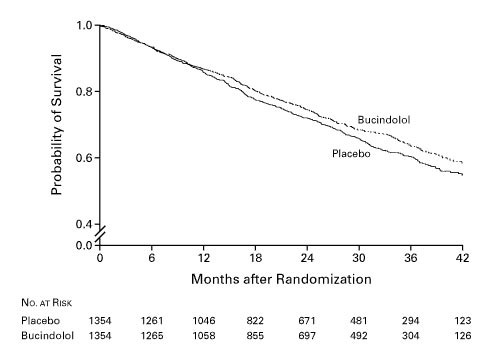
There were a total of 860 deaths, 449 in the placebo group and 411 in the bucindolol group (log-rank statistic=1.63; unadjusted P=0.10).
WASHINGTON — Beta blockers have been shown to improve outcomes in patients with heart failure with reduced ejection fraction (HFrEF), a complex syndrome characterized by impairment of the left ventricle resulting in a low left ventricular ejection fraction (LVEF).
A measure of the heart’s ability to pump blood to the body’s tissues and organs, LVEF is routinely assessed by various imaging techniques in patients with heart failure. However, nearly half of all patients with HFrEF also have reduced right ventricular ejection fraction (RVEF), often defined as <35%, and those with RVEF <20% are at the highest risk for adverse outcomes.
Patients are often risk-stratified based on LVEF to optimize therapy, but less was known about whether the effect of beta blockers in patients with HFrEF varied by RVEF.
To better understand whether the presence of RVEF influenced the effectiveness of beta blockers in patients with HFrEF, researchers from the Washington, DC, VAMC, led by Phillip H. Lam, MD, conducted a post-hoc analysis of the Beta-Blocker Evaluation of Survival Trial (BEST), a randomized placebo-controlled trial of bucindolol, a nonselective beta-adrenergic blocker with vasodilator properties, in patients with HFrEF (LVEF of 35%).
Between 1995 and 1998, 2708 patients were randomized to either bucindolol or placebo. When the trial was terminated prematurely after two years owing to the “totality of evidence regarding the usefulness of beta-blocker treatment derived from BEST and other studies,” the effect of bucindolol on the primary endpoint of all-cause mortality had not reached statistical significance.
Of the 2,798 patients in BEST, 2,008 had data on baseline RVEF. Of those, 1,012 had an RVEF of less than 35% (the median value), and 996 had an RVEF of 35% or greater. In the current study, which was published in January 2022 in the Journal of Cardiac Failure, the official journal of the Heart Failure Society of America, the researchers examined whether the effect of bucindolol varied by RVEF. As in the BEST trial, the primary outcome was all-cause mortality. Secondary outcomes included cardiovascular death, sudden cardiac death, death owing to pump failure, all-cause hospitalization and HF hospitalization.1
The Key Findings
- The effect of bucindolol on all-cause mortality in 2008 patients with baseline RVEF is consistent with that in the 2798 patients in the main trial. In the subgroup of 1012 patients with a baseline RVEF of 35% or greater, patients in the bucindolol group (vs. placebo) had a lower risk of all-cause mortality that was statistically significant and not observed in the subgroup of 996 patients with a baseline RVEF of less than 35% .
- Randomization to bucindolol was associated with a 32% and 28% significantly lower risk of all-cause mortality in the upper two RVEF quartiles (45% or greater and 35% to 44%), a 14% nonsignificantly lower risk in the RVEF quartile 25% to 34%, and a 20% nonsignificantly higher risk in the quartile less than 25%
- The baseline RVEF also modified the effect of bucindolol on cardiovascular mortality and sudden cardiac death but not of other outcomes.
“The findings from the current study demonstrate that baseline RVEF may modify the effect of bucindolol on mortality, cardiovascular mortality and sudden cardiac death in patients with HFrEF,” Lam said. In addition to his work at the DCVAMC, Lam is an Advanced Heart Failure and Transplant Cardiology physician at the MedStar Heart & Vascular Institute at MedStar Washington Hospital Center.
Benefits in RVEF 35% or Greater
“The beneficial effects of bucindolol were only significant in the subgroup with an RVEF of 35% or greater but not in the subgroup with an RVEF of less than 35%, who represented one-half of the patients,” he added.
To the authors’ knowledge, no prior studies have examined whether the effect of beta blockers in patients with HFrEF is modified by the RVEF.
Lam said it was a bit surprising to researchers that those with an RVEF 35% or greater responded to bucindolol and not those with RVEF less than 35%, because treatment effects are often greater in subgroups of high-risk patients. The group had previously demonstrated that a reduced RVEF in patients with HFrEF was associated with poor outcomes.
“Because bucindolol is not approved for use in patients with HFrEF, the results of our study may not be generalizable to beta blockers approved and recommended for use in HFrEF, namely carvedilol, metoprolol succinate and bisoprolol,” explained Ali Ahmed, MD, MPH, the study’s senior author and associate chief of staff for health and aging at the DCVAMC. A geriatrician who specializes in heart failure in older adults, Ahmed added, “However, if these hypothesis-generating findings from our study can be replicated using approved beta blockers, they would provide evidence to guide patient selection for optimal clinical benefit from beta blockers in this population.”
- Lam PH, Keramida K, Filippatos GS, Gupta N, et. al. J Card Fail. 2022 Jan;28(1):65-70. doi: 10.1016/j.cardfail.2021.07.026. Epub 2021 Aug 19. PMID: 34419597.
- A trial of the beta blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001 May 31;344(22):1659-67. doi: 10.1056/NEJM200105313442202. PMID: 11386264.


