Technologist Robert Jacobse operates the CT scanner at the Durham, NC, VAMC. The scans are part of VA’s comprehensive lung cancer screening program. Photo by Linnie Skidmore
ANN ARBOR, MI — Veterans who underwent VHA lung cancer screening were more likely to be diagnosed at an earlier stage and had higher cure rates than those who were not screened, according to a new observational study.
A report in the journal Cancer noted that current recommendations call for annual lung cancer screening of adults 50–80 years old with at least a 20-pack-year smoking history who currently smoke or have quit within the past 15 years.1
The research led by the Ann Arbor, MI, VA Healthcare System sought to provide real-world effectiveness of imaging to detect lung cancer. To do that, they focused on VHA patients diagnosed with lung cancer from 2011 to 2018.
Among 57,919 veterans diagnosed with lung cancer, 2,167 (3.9%) underwent screening before diagnosis. Those who underwent screening were found to have higher rates of early (stage I) diagnoses compared with those who had no screening (52% vs. 27%), lower rates of death from any cause (49.8% vs. 72.1%) and death from cancer (41.0% vs. 70.3%) over 5 years.
“It is incredible to witness how dedicated national efforts to increase lung cancer screening from the Lung Precision Oncology Program can lead to substantial improvements in lung cancer outcomes,” said co-corresponding author Michael Green, MD, PhD, who also is at the University of Michigan.
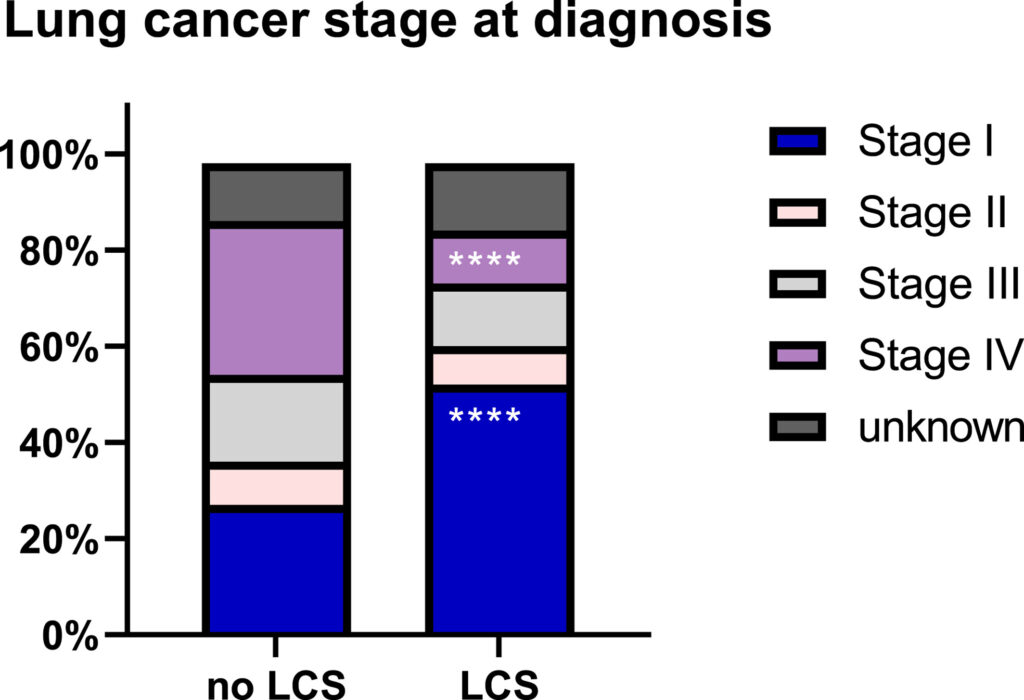
Click to Enlarge: Stage migration of lung cancer diagnoses between 2011 and 2018 among screened and unscreened patients. ****p < .0001 via the χ2 test. LCS indicates lung cancer screening. Source: American Cancer Society
The VA is the largest integrated provider of cancer care in the United States and provides healthcare for about 6.7 million veterans a year systemwide. Because of a greater prevalence of smoking among veterans with military service, the cohort has an estimated 76% higher age-specific incidence of lung cancer than the general population. The Lung Cancer Screening Demonstration Project estimated that almost 900,000 VA patients would be eligible for LCS, but, until this study, there had been limited VA data beyond single-site reports to assess lung cancer screening effectiveness to diagnosis malignancy at an earlier stage.
“Despite randomized trials demonstrating a mortality benefit to low-dose computed tomography screening to detect lung cancer, uptake of lung cancer screening (LCS) has been slow, and the benefits of screening remain unclear in clinical practice,” the researchers wrote.
They said their large national study corroborates the value of lung cancer screening in clinical practice and called for efforts to widely adopt the “vital intervention.”
They added, “Among screening-eligible patients who underwent National Comprehensive Cancer Network guideline-concordant treatment, screening resulted in substantial reductions in all-cause mortality (adjusted hazard ratio [aHR], 0.79; 95% confidence interval [CI], 0.67-0.92; p =0.003) and lung-specific mortality (aHR, 0.61; 95% CI, 0.50–0.74; p < 0.001).”
The leading cause of cancer deaths worldwide, lung cancer is usually diagnosed at an advanced stage, according to background information in the article. Among patients with nonsmall cell lung cancer (NSCLC), the most common form of lung cancer, 5-year survival for early stage I disease is about 68%, compared to a rate of 5.8% for metastatic disease.
Prompt Detection
“Thus, prompt detection of early-stage disease is paramount to improving outcomes,” the authors pointed out, adding that the U.S. Preventive Services Task Force (USPSTF) currently recommends annual low-dose computed tomography (LDCT) screening for adults between the ages of 50 and 80 years with at least a 20-pack-year smoking history who currently smoke or have quit within the past 15 years. This recommendation is based on randomized trial evidence of a mortality benefit and stage migration effect with screening.
Past research trials showed substantial decreases in lung cancer mortality related to lung cancer screening (LCS), the report advised, a 20% decline in mortality in The National Lung Screening Trial (NLST) and a similar drop in the Dutch-Belgian Randomized Lung Cancer Screening Trial lung screening trial.
“Notably, a higher proportion of screening-detected lung cancers were stage I compared to non–screening-detected cases (58.6% vs. 14.2%),” the researchers wrote. “Presumably, the concept of stage migration, or the shift in identifying more early stage cancers at the time of diagnosis before progression to advanced disease, contributed to the mortality benefit in these trials.”
They added, “However, the mortality benefits of screening shown in these trials can only be realized with broad implementation of screening into clinical practice, and there remains a need to demonstrate the real-world effectiveness of LCS. There are patient-, provider-, and system-specific challenges to the implementation of screening, which have resulted in the slow adoption of screening in clinical care.”
Further, the report described how rates of adherence to screening, which were greater than 90% in both randomized trials, generally are much lower in real-world settings, especially among Black patients and current smokers, as well as younger or less-educated patients. In fact, the study cited a recent analysis from the LCS registry demonstrating that only 1.9% of 7.6 million eligible smokers per NLST criteria underwent screening in 2016.
Because participants in randomized controlled trials are often more educated or healthier than the general population, questions have been raised about the applicability of trial results to the general population; those often more elderly or frail or have more medical comorbidities. “It is also possible that the implementation of screening into practice has been affected by provider concern for overdiagnosis and the treatment of cancers that would have otherwise remained indolent,” the researchers suggested. “Additional concerns involve adequate infrastructure available to support comprehensive screening programs, as noted in a survey of pulmonologists in the Veterans Health Administration (VA) system.”
- Edwards DM, Pirzadeh M, Van T, Jiang R, et.al. Impact of lung cancer screening on stage migration and mortality among the national Veterans Health Administration population with lung cancer. Cancer. 2024 Jun 10. doi: 10.1002/cncr.35340. Epub ahead of print. PMID: 38853532.

