Label Update Opens Way for Use of Keytruda/Lenvima Combination
LA JOLLA, CA — When the law, widely known as the PACT Act, was signed into law two years ago this month, kidney cancer became a presumptive condition for VA care for the first time.
For veterans who have been exposed to toxic substances, such as burn pits or particulate matter, coverage is now available for renal cell carcinoma, including chromophobe, clear cell, clear cell papillary, collecting duct, medullary, papillary and unclassified types – in other words, both clear cell and non-clear cell types. Non-renal cell carcinoma, including renal sarcoma and Wilms tumor, are also on the list.
The National Cancer Institute has reported that per- and polyfluoroalkyl substances (PFAS), including perfluorooctanoic acid (PFOA), a diverse class of synthetic chemicals used in commercial and industrial applications, are linked to kidney cancer development. Burn pits have the potential to release to the environment heavy metals, perchlorate, particulate matter, per- and polyfluoroalkyl substances (PFAS), dioxins/furans, explosive compounds, and other toxic and hazardous contaminants and are highly persistent in the environment, according to the Environmental Protection Agency.
Much of the research on kidney cancer focuses on clear cell renal cell carcinoma, which is, by far, the most common type. But important advances are occurring with non-clear cell renal cell carcinoma (nccRCC), as well. A recent review from the University of California, San Diego discussed nccRCC, which makes up about a fourth of all kidney cancer cases.
“As one of the most prevalent cancer types in the United States, kidney cancer is a major malignancy, comprising an estimated 5% and 3% of all new cancer cases in the United States in males and females, respectively,” the authors wrote in Therapeutic Advances in Urology. “Among cancers of the kidney, RCC is the most common type, making up over 90% of kidney cancer cases. RCC can further be divided into two subgroups, with the majority being ccRCC (75%), and the remaining being nccRCC (25%).”1
The researchers pointed out that the rare group of nccRCC malignancies can further be subdivided into the following histologic subtypes:
- papillary,
- oncocytic and chromophobe,
- collecting duct,
- molecularly defined renal carcinomas (which include SMARCB1-deficient medullary RCC, translocation,
- hereditary RCCs), and
- other renal tumors.
Papillary RCCs (pRCC make up 10–15% of all RCCs and “arise from a nephron’s proximal and distal convoluted tubules,” according to the report, which added, “Historically, pRCC has been divided into Type 1 and Type 2; however, the World Health Organization 2022 classification eliminated the Type 1/2 pRCC subcategorization, given the recognition of frequent mixed tumor phenotypes and different molecular underpinnings across the subtypes.”
The next most frequent variant histology is chromophobe RCC (chRCC) for 5–7% of all RCCs and originates from the distal nephron unlike ccRCC, which arises from the proximal nephron.
Collecting duct RCC (cdRCC) occurs in the distal collecting duct epithelium and represents 1–2% of RCCs, according to the authors, who added, “Finally, unclassified or other renal tumors comprise approximately 2–6% of RCCs. Other rare RCC subtypes represent <1% of all RCC tumors, including SMARCB1-deficient medullary RCC, which arises from the calyceal epithelium, and molecularly defined RCC variants, such as TFE3-rearranged, transcription factor EB (TFEB)-rearranged, TFEB-amplified, FH-deficient, succinate dehydrogenase (SDH)-deficient, anaplastic lymphoma kinase (ALK)-rearranged, ELOC-mutated, and other SMARCB1 (INI1)-deficient RCCs.”
Treatment for nccRCC often involves agents used in the more common clear-cell type, according to the review.
Recently, Eisai announced a label update for its lenvatinib product, marked as Lenvima. Labeling in the United States now includes clinical efficacy data for the first-line treatment of advanced non-clear cell renal cell carcinoma (nccRCC) for the orally available multiple receptor tyrosine kinase inhibitor discovered by Eisai.
The update is based on data from KEYNOTE-B61, a Phase 2, single-arm trial evaluating pembrolizumab, Merck’s anti-PD-1 therapy marketed as Keytruda, plus Lenvima for the first-line treatment of adult patients with advanced nccRCC.
“While the label now includes efficacy data on the non-clear cell population, the approved indication for KEYTRUDA plus LENVIMA for the first-line treatment of adult patients with advanced RCC is unchanged,” according to the press release, which noted that Merck received an updated label for Keytruda as well.
Eisai pointed out that Keytruda plus Lenvima is the first and only immunotherapy and tyrosine kinase inhibitor (IO plus TKI) combination with data in both clear cell and non-clear cell advanced RCC in the FDA-approved label.
The trial leading to the change, KEYNOTE-B61, was a Phase 2, single-arm, multicenter study that enrolled 160 patients with previously untreated nccRCC to investigate an immune checkpoint inhibitor in combination with a TKI. Defined as the major efficacy outcome measure was objective response rate (ORR) as assessed by blinded independent central review (BICR) using Response Evaluation Criteria in Solid Tumors (RECIST 1.1). In addition, efficacy outcomes measured included duration of response (DOR) as assessed by BICR using RECIST 1.1.
The press release advised that the ORR was 51% (95% CI, 43-59), with a complete response rate of 8% and a partial response rate of 42%, for patients who received Keytruda and Lenvima, while the median DOR was 19.5 months (range: 1.5+, 23.5+ (+ denotes ongoing response)). No new safety signals were observed.
Earlier this year, an article in the Journal of Clinical Oncology presented the final prespecified overall survival (OS) analysis of the open-label, phase III CLEAR study in treatment-naïve patients with advanced renal cell carcinoma (aRCC). With an additional follow-up of 23 months from the primary analysis, the Memorial Sloan-Kettering-led researchers wrote that the OS hazard ratio (HR) was 0.79 (95% CI, 0.63 to 0.99), while the median OS (95% CI) was 53.7 months (95% CI, 48.7 to not estimable [NE]) with lenvatinib plus pembrolizumab vs. 54.3 months (95% CI, 40.9 to NE) with sunitinib; 36-month OS rates (95% CI) were 66.4% (95% CI, 61.1 to 71.2) and 60.2% (95% CI, 54.6 to 65.2), respectively.2
At the same time, the median progression-free survival (95% CI) was 23.9 months (95% CI, 20.8 to 27.7) with lenvatinib plus pembrolizumab and 9.2 months (95% CI, 6.0 to 11.0) with sunitinib (HR, 0.47 [95% CI, 0.38 to 0.57]).
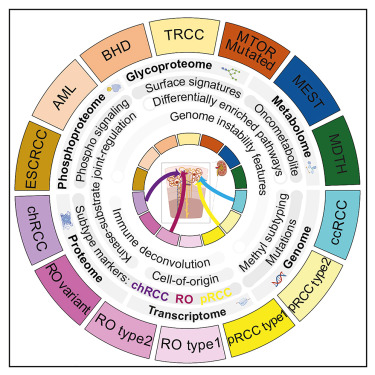
Recently, a study in Cell Reports Medicines showed promise for helping pharmaceutical companies develop additional effective treatments for nccRCC in the future. The study led by University of Michigan Health Rogel Cancer Center researchers identified novel biomarkers in renal cell carcinomas.3
“Non-clear cell renal cell carcinomas (non-ccRCCs) encompass diverse malignant and benign tumors,” the authors wrote. “Refinement of differential diagnosis biomarkers, markers for early prognosis of aggressive disease, and therapeutic targets to complement immunotherapy are current clinical needs.”
The study involved analyses of 48 non-ccRCCs compared with 103 ccRCCs, revealing proteogenomic, phosphorylation, glycosylation, and metabolic aberrations in RCC subtypes. Researchers said the study expanded their knowledge of proteogenomic signatures, biomarkers, and potential therapeutic targets in non-ccRCC.
“Despite having different molecular make-ups, non-ccRCCs are treated with the standard of care devised for the common form, affecting treatment outcomes,” according to a U-M press release. “Differential diagnosis of non-ccRCC tumors can be challenging due to overlapping morphological features and a lack of specificity in current biomarkers.”
But “the standard of care for non-ccRCCs is evolving,” said Saravana Mohan Dhanasekaran, PhD, an associate research scientist at the Michigan Medicine Center for Translational Pathology who helped lead the study.
“Rare cancers are often left out from major profiling efforts, so therapeutic and diagnostic advances in this space have been limited. Until now, no single center has had enough samples of the quality needed for comprehensive multi-omics profiling, as we’ve carried out in this study,” Dhanasekaran said.
- Naik P, Dudipala H, Chen YW, Rose B, Bagrodia A, McKay RR. The incidence, pathogenesis, and management of non-clear cell renal cell carcinoma. Ther Adv Urol. 2024 Feb 29;16:17562872241232578. doi: 10.1177/17562872241232578. PMID: 38434237; PMCID: PMC10906063.
- Robert J. Motzer et al. Lenvatinib Plus Pembrolizumab Versus Sunitinib in First-Line Treatment of Advanced Renal Cell Carcinoma: Final Prespecified Overall Survival Analysis of CLEAR, a Phase III Study. JCO 42, 1222-1228(2024). DOI:10.1200/JCO.23.01569
- Li GX, Chen L, Hsiao Y, Mannan R, Clinical Proteomic Tumor Analysis Consortium, et. Al. Comprehensive proteogenomic characterization of rare kidney tumors. Cell Rep Med. 2024 May 21;5(5):101547. doi: 10.1016/j.xcrm.2024.101547. Epub 2024 May 3. PMID: 38703764; PMCID: PMC11148773.