Research shows women may be at greater risk of developing dementia, including Alzheimer’s disease. In the military, this affects the female veteran population. Photo credit: U.S. Army Master Sgt. Timothy Lawn
Leqembi Given Fast Track Approval by FDA
SILVER SPRING, MD—Now that the Food and Drug Administration has granted Fast Track approval for the new Alzheimer’s disease medication Leqembi, the issue of who will pay for it looms large. The VA is expected to be prominent in that discussion.
In a recent press release, the lead company developing the drug, Eisai R&D Management Co., Ltd., noted that it looked “forward to continuing to engage constructively with various payors, including the Centers for Medicare and Medicaid (CMS), TRICARE, the U.S. Veteran’s Health Administration and private health insurance companies to ensure appropriate beneficiaries have access to this new therapy.’”
The press release noted that Medicare patients currently do not have access to Leqembi, although Medicaid sole beneficiaries who are diagnosed by a healthcare professional with mild cognitive impairment or mild dementia stage of disease and have confirmed presence of amyloid plaque in the brain will have access to the drug under the Medicaid program post accelerated approval, depending on individual state processes.
The drug was expected to be available by the end of the third week of January. The approval of Leqembi was granted to Eisai R&D Management Co., Ltd., which worked with Biogen Inc. to develop lecanemab-irmb. The company said it decided to initially price the drug at $26,500 per year for the wholesale acquisition cost, which is below the quantified societal value.
“Leqembi is the second of a new category of medications approved for Alzheimer’s disease that target the fundamental pathophysiology of the disease. These medications represent an important advancement in the ongoing fight to effectively treat Alzheimer’s disease,” according to a press release from the Food and Drug Administration (FDA). The application was given Fast Track, Priority Review and Breakthrough Therapy designations.
“Alzheimer’s disease immeasurably incapacitates the lives of those who suffer from it and has devastating effects on their loved ones,” explained Billy Dunn, MD, director of the Office of Neuroscience in the FDA’s Center for Drug Evaluation and Research. “This treatment option is the latest therapy to target and affect the underlying disease process of Alzheimer’s, instead of only treating the symptoms of the disease.”
The Accelerated Approval pathway allows the FDA to OK drugs for serious conditions when there is an unmet medical need and “a drug is shown to have an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit to patients,” according to an FDA press release.
The estimated number (prevalence) of VA enrollees with Alzheimer’s dementia in fiscal year 2021, the most recent data available, was 251,711. That was a slight, 0.8% increase from FY2020. The VA has projected, however, that the projected number (prevalence) of VA enrollees with Alzheimer’s dementia in FY2033 will be 319,869. That represents a 27.1% increase—another 68,158 enrollees—from the estimated number in FY2021.
The number of U.S. veterans overall with Alzheimer’s—not just those enrolled for VA care—estimated at 457,391 and is expected to rise to 488,353 by FY2033.
In a study published in the New England Journal of Medicine, researchers suggested that the accumulation of soluble and insoluble aggregated amyloid-beta (Aβ) might initiate or potentiate pathologic processes in Alzheimer’s disease. The Yale School of Medicine-led authors pointed out that lecanemab, a humanized IgG1 monoclonal antibody that binds with high affinity to Aβ soluble protofibrils, is being tested in patients with early Alzheimer’s disease.1
Their 18-month, multicenter, double-blind, Phase 3 trial involved patients 50 to 90 years of age with early Alzheimer’s disease, defined as mild cognitive impairment or mild dementia due to Alzheimer’s disease, with evidence of amyloid on positron-emission tomography (PET) or by cerebrospinal fluid testing.
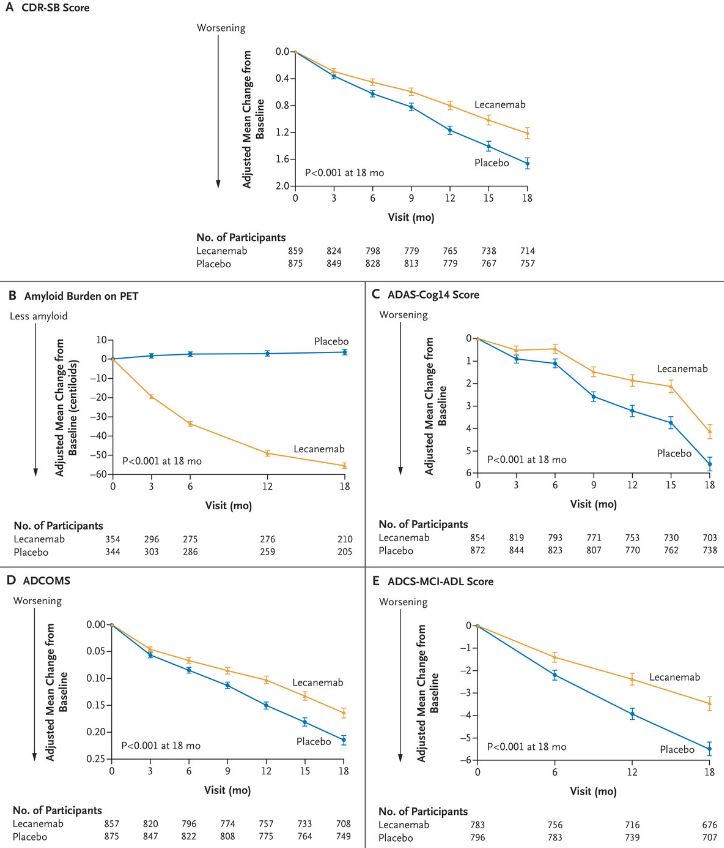
Researchers randomly assigned 1,795 participants in a 1:1 ratio to receive intravenous lecanemab (10 mg per kilogram of body weight every 2 weeks) or placebo. The change from baseline at 18 months in the score on the Clinical Dementia Rating Sum of Boxes (CDR-SB; range, 0 to 18, with higher scores indicating greater impairment) was considered the primary endpoint. Secondary endpoints of importance were the change in amyloid burden on PET, the score on the 14-item cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog14; range, 0 to 90; higher scores indicate greater impairment), the Alzheimer’s Disease Composite Score (ADCOMS; range, 0 to 1.97; higher scores indicate greater impairment), and the score on the Alzheimer’s Disease Cooperative Study-Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS-MCI-ADL; range, 0-53; lower scores indicate greater impairment).
The researchers reported that the mean CDR-SB score at baseline was approximately 3.2 in both groups, adding that results indicate that the adjusted least-squares mean change from baseline at 18 months was 1.21 with lecanemab and 1.66 with placebo (difference, -0.45; 95% confidence interval [CI], -0.67 to -0.23; P<0.001).
“In a substudy involving 698 participants, there were greater reductions in brain amyloid burden with lecanemab than with placebo (difference, -59.1 centiloids; 95% CI, -62.6 to -55.6),” according to the report. “Other mean differences between the two groups in the change from baseline favoring lecanemab were as follows: for the ADAS-cog14 score, -1.44 (95% CI, -2.27 to -0.61; P<0.001); for the ADCOMS, -0.050 (95% CI, -0.074 to -0.027; P<0.001); and for the ADCS-MCI-ADL score, 2.0 (95% CI, 1.2 to 2.8; P<0.001). Lecanemab resulted in infusion-related reactions in 26.4% of the participants and amyloid-related imaging abnormalities with edema or effusions in 12.6%.”
The authors conclude that lecanemab “reduced markers of amyloid in early Alzheimer’s disease and resulted in moderately less decline on measures of cognition and function than placebo at 18 months but was associated with adverse events.
The study team is calling for longer trials to determine the efficacy and safety of lecanemab in early Alzheimer’s disease.
The recommended dosage of the drug is 10 mg/kg administered intravenously once every two weeks to eligible patients with confirmed presence of Aβ pathology prior to initiating treatment. Prescribing information for Leqembi includes a warning for amyloid-related imaging abnormalities (ARIA), which are known to occur with antibodies of this class.
Eisai said it is developing a multifaceted educational initiative to further advance the understanding in the Alzheimer’s disease healthcare community about the real-world management and monitoring of
According to the FDA, treatment with Leqembi should be initiated in patients with mild cognitive impairment or mild dementia stage of disease. No safety or effectiveness data on initiating treatment at earlier or later stages of the disease than were studied is available.
- van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, et. al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023 Jan 5;388(1):9-21. doi: 10.1056/NEJMoa2212948. Epub 2022 Nov 29. PMID: 36449413.