Matthew Rettig, MD, the chief of hematology and oncology at the VA Greater Los Angeles Health Care System
LOS ANGELES — That men tend to have worse outcomes with COVID-19 has been observed since the beginning of the novel coronavirus outbreak.
In May, an Italian-led study published online by Annals of Oncology began to offer some clues as to why that might be the case. The authors observed that men being treated for prostate cancer with androgen-deprivation therapies were less likely to develop COVID-19 and to have less-severe cases if they got it.1
That observational study focused on 4,532 men infected with COVID-19, of which 9.5% (430) had cancer and 2.6% (118) had prostate cancer. Results suggested that male cancer patients had a 1.8-fold increased risk of COVID-19 infection out of the whole male population and developed more severe disease. Looking only at prostate cancer patients in the Veneto region of Italy, however, the study team found that only four out of 5,273 men on ADT developed COVID-19 infection, and none of them died. This compared to 37,161 men with prostate cancer who were not receiving ADT, of whom 114 developed COVID-19 and 18 died. Among 79,661 patients with other types of cancer, 312 developed COVID-19 and 57 died.
“Patients with prostate cancer receiving androgen-deprivation therapies had a significant fourfold reduced risk of COVID-19 infections compared to patients who did not receive ADT,” explained lead researcher Andrea Alimonti, MD, of Università della Svizzera Italiana. “An even greater difference was found, when we compared prostate cancer patients receiving ADT to patients with any other type of cancer; there was a more than fivefold reduction in risk of infection among the prostate cancer patients on ADT.”
That study was observational, however, and the authors called for clinical trials to confirm their findings. That’s where a leading VA researcher stepped in.
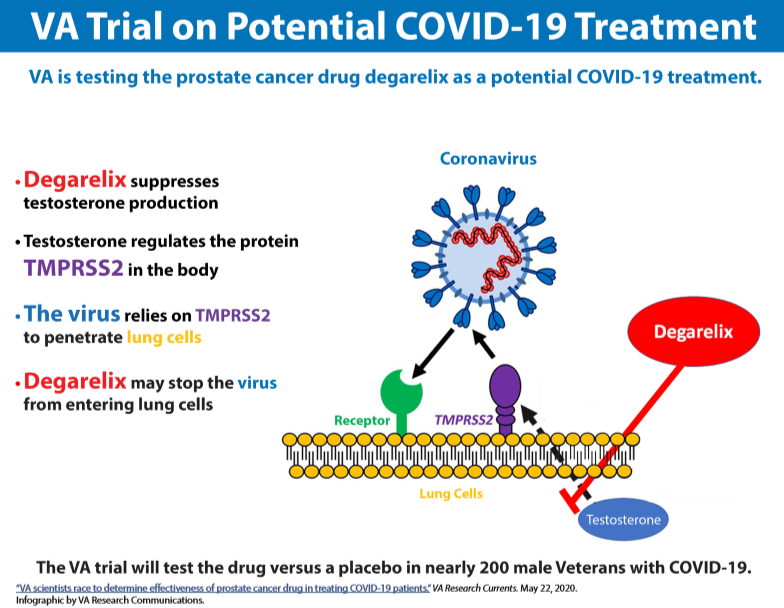
Matthew Rettig, MD, the chief of hematology and oncology at the VA Greater Los Angeles Health Care System, is heading a clinical trial to investigate a prostate cancer drug as a potential treatment for male veterans with COVID-19. In a double-blind randomized controlled study, he and his colleagues are comparing the drug degarelix, marketed as Firmagon, to placebo for improving the clinical outcomes of nearly 200 veterans who have been hospitalized with novel coronavirus.
The male hormone testosterone can fuel the spread of prostate cancer, but degarelix rapidly but temporarily suppresses the body’s production of testosterone, which regulates the protein TMPRSS2. The virus that causes COVID-19, a respiratory disease, relies on TMPRSS2 to penetrate lung cells. By using degarelix, Rettig said he and his team anticipate that they can reduce the production of TMPRSS2 in lung tissue and prevent the virus from entering lung cells.
Degarelix was one of the hormone suppression drugs given to patients in the Italian study, along with Lupron, marketed as Leuprolide. Rettig told VA’s Research Currents that results of the earlier study bolster his belief that degarelix may be effective in treating COVID-19.
Degarelix was chosen over other drugs for the HITCH trial because it’s the only one that rapidly drops male hormone levels, he noted, adding, “On average, hormone levels drop about 90% in 24 hours with degarelix, whereas, with all of the other ADT drugs, it can take a few weeks to reduce testosterone levels. We don’t have the luxury of time when we’re talking about sick COVID patients who are hospitalized. So we chose degarelix. It works rapidly, and it’s temporary, and the effects on testosterone levels, as well as the side effects, resolve in just a few weeks. Most, if not all, of the side effects of degaralix are attributable to its long-term use for prostate cancer.”
Coordinators of the trial are in the process of also recruiting the VA hospitals in Chicago, Philadelphia, and Bronx, NY.
The trial will focus on veterans who are moderately ill with COVID-19. “We think that population is really suffering from an overactive immune response to the virus,” Rettig pointed out. “Therefore, the therapy we’re using in the trial, which aims to reduce the virus and its entry into the human lung tissue, probably will not be relevant in patients who are the most severely ill. We’re trying to narrow this down to men who are sick enough to require treatment but not sick enough to be in the ICU.”
Women will not be participants in the trial, because evidence exists that degarelix could have the opposite effect in the female body by increasing TMPRSS2 production and thus worsening the severity of COVID-19 symptoms.
“Male hormones and female hormones seem to have different or opposing effects for COVID-19,” Rettig explains. “We think male hormones may be driving the susceptibility and severity of COVID-19, whereas female hormones [estrogen] seem to suppress the severity of the disease.”
-
Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, Carbone GM, Cavalli A, Pagano F, Ragazzi E, Prayer-Galetti T, Alimonti A, Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (n=4532), Annals of Oncology (2020), doi: https://doi.org/10.1016/j.annonc.2020.04.479. This is a PDF file of an article that has undergone enhancements after