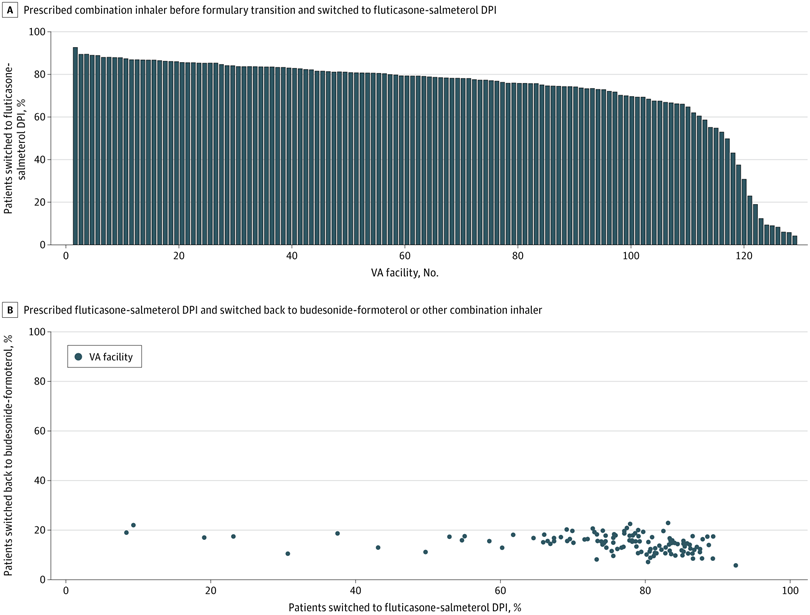
Click to Enlarge: Patients Switched to a Fluticasone-Salmeterol Dry-Powder Inhaler (DPI) by US Department of Veterans Affairs (VA) Facility and Patients Switched Back to Budesonide-Formoterol or Another Combination Inhaler Following the VA National Formulary Transition
Although the VA formulary is determined centrally, it is implemented locally at the facility level. A. Proportion of patients prescribed a combination inhaler prior to the formulary transition who were switched to the fluticasone-salmeterol DPI (Wixela Inhub; Viatris) following the VA national formulary transition in July 2021. Each bar represents 1 VA facility. B. Proportion of patients prescribed the fluticasone-salmeterol DPI who were switched back to budesonide-formoterol (Symbicort; AstraZeneca) or another combination inhaler. Each dot represents 1 VA facility. Most VA facilities switched more than 80% of patients receiving combination inhalers to the fluticasone-salmeterol DPI, but a small proportion of facilities had much lower switch rates. Regardless of switch rate, however, 14.2% of patients were switched back to an alternate inhaler. Source: JAMA Network Open
ANN ARBOR, MI — The VA’s experience provided valuable information on what is likely to occur when a formulary changes for environmental reasons, according to a new report.
More than 3 years ago, in July 2021, the VA transitioned from the budesonide-formoterol MDI (Symbicort; AstraZeneca) to the fluticasone-salmeterol DPI (Wixela Inhub; Viatris) as its preferred combination inhaled corticosteroid and long-acting β2-agonist controller medication.
A new study in JAMA Network Open analyzed trends in inhaler-prescribing patterns among VA facilities following the national formulary transition, which came about because metered-dose inhalers (MDIs) containing hydrofluorocarbon propellants have a greater carbon footprint than dry-powder inhalers (DPIs), which do not require an aerosol propellant for drug delivery.1
“Despite calls to reduce inhaler-related greenhouse gas emissions, data on the large-scale implementation of device switching from MDIs to DPIs are limited, following a competitively bid contract negotiation,” wrote the authors from the VA Ann Arbor, MI, Healthcare System, the University of Michigan and the VA Pharmacy Benefit Management Services in Hines, IL.
The study team extracted data on inhaler dispensing from Jan. 1, 2018, to Dec. 31, 2022, from the VA Corporate Data Warehouse. Of 347,486 patients with a mean age of 65.8, 74.9% were switched to the fluticasone-salmeterol DPI. Of these switched patients, 37 036 (14.2%) were subsequently prescribed a non–fluticasone-salmeterol combination inhaler, the researchers pointed out.
The study added that, among 23,350 VA nonformulary consultations, there were 10,481 requests for budesonide-formoterol and 12,869 requests for other combination inhalers. Of those, 8,713 (83.1%) and 12,628 (98.1%) were approved, respectively.
“The most common reason for nonformulary consultation approval was a therapeutic failure of the fluticasone-salmeterol DPI, and the most common reason for disapproval was that a trial of a preferred alternative device had not been exhausted,” the researchers wrote.
The study team added, “A VA national formulary transition in 2021, favoring a DPI (fluticasone-salmeterol) over an MDI (budesonide-formoterol), resulted in marked changes in inhaler prescribing across the VA health care system, with swift uptake of the DPI among a majority of facilities. Following implementation of this formulary change, fewer than 1 in 6 patients (37 036 of 260 268 [14.2%]) were switched back to budesonide-formoterol or another nonformulary combination inhaler, and the rate of switchback was consistent across VA facilities.”
Limitations of the study included that, while most veterans who have filled a medication through the VA rely on the VA alone for prescription medication, prescribing outside of the healthcare system was not considered and could have led to an underestimation of inhaler device switching after the formulary transition.
“As health care systems and payers consider substituting MDIs with DPIs to mitigate the climate impact of respiratory care, our results suggest that, in a large sample of veterans with a high burden of respiratory disease, a device transition may be carried out with a relatively low failure rate (ie, requiring a transition back to an MDI),” the authors advised.
They said that additional research is needed to understand the effects of inhaler device switching on respiratory health outcomes, healthcare use and net health care-related greenhouse gas emissions, as well as optimal methods to support a successful device transition.
- Rabin AS, Weinstein JB, Whittington TN, et al. Implementation of a Metered-Dose Inhaler to Dry-Powder Inhaler National Formulary Transition. JAMA Netw Open. 2024;7(12):e2449234. doi:10.1001/jamanetworkopen.2024.49234

