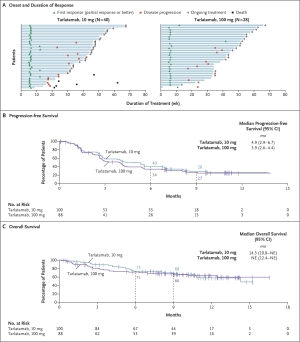
Click to Enlarge: Antitumor Activity of Tarlatamab.
Panel A shows the time to response, the duration of response, and patient status as of the data cutoff date for all the patients who were assessed as having an objective response (complete or partial response; primary end point) to 10 mg or 100 mg of tarlatamab, as assessed by blinded independent central review according to the Response Evaluation Criteria in Solid Tumors, version 1.1. Panel B shows the Kaplan–Meier curve of progression-free survival in the analysis population for antitumor activity, which included 100 patients who had been assigned to receive 10 mg of tarlatamab in part 1 or part 2 of the trial and 88 patients who had been assigned to receive 100 mg of tarlatamab in part 1 of the trial. Panel C shows the Kaplan–Meier curve of overall survival in the analysis population for antitumor activity. The tick marks in Panels B and C indicate censored data. NE denotes not evaluable. Source:
SILVER SPRING, MD — Small cell lung cancer (SCLC) is a highly aggressive form of lung cancer, characterized by rapid growth and early metastasis. Accounting for roughly one in seven lung cancers, SCLC presents unique challenges in treatment due to its biologically aggressive nature and the rapid development of resistance to therapies.
For more than four decades, SCLC treatment remained largely static, relying on largely on platinum-based chemotherapy to which patients typically respond very well initially and nearly always relapse. Consequently, mortality rates have remained stunningly poor, with most patients dying within two years. Recent advances in immunotherapy and targeted therapies in the last few years have provided new hope for improving outcomes, especially in the relapsed or metastatic setting.
The combination of cisplatin or carboplatin with etoposide remains the cornerstone of SCLC treatment. In limited-stage disease, in which tumors have remained in the hemithorax of origin or spread only to the mediastinum or supraclavicular lymph nodes, platinum therapy, etoposide and concurrent chemoradiotherapy (CCRT) provides an objective response rate as high as 94.8%, as demonstrated in the control arm of the STIMULI trial.1 The trial reported two-year and three-year overall survival rates of 66.4% and 49.3%, respectively, showcasing the impressive initial responses and representing a very substantial increase in overall survival compared to the median overall survival (OS) of 10.3 months and 18-month OS of 25% seen with just chemotherapy and etoposide.2
In addition to CCRT, prophylactic cranial irradiation is commonly used to prevent the development of brain metastases, which are present in 10% to 25% of SCLC patients at diagnosis and arise in up to half as the cancer progresses.
A small subset of patients with limited-stage SCLC may benefit from surgery, recent research found. Using data from patients with stage 1 SCLC from the National VA Cancer Care Cube Registry, researchers compared patients who had undergone surgery (223) with or without chemotherapy, radiation or a combination to those who had concurrent chemoradiotherapy (CRT). Median overall survival for patients whose treatment included surgery reached 3.87 years vs. 2.45 years median overall survival for the CRT cohort. The improvement was independent of the location of the tumor.3
Unfortunately, despite these high response rates, relapse remains common, reflecting the paradox of SCLC—the bright promise of its initial chemosensitivity belies its fleeting response.
Increasingly, however, researchers have focused on immunotherapy as a novel means to both control and extend survival in SCLC, especially in the relapsed and extensive-stage settings.
Immunotherapy: A New Frontier in SCLC Treatment
In the extensive-stage setting, standard of care includes a combination of platinum-based chemotherapy and immune checkpoint inhibitors (ICIs), such as atezolizumab or durvalumab. This has been particularly beneficial for veterans with extensive-stage SCLC, who often have comorbidities that limit treatment options and who are more prone to early recurrence of disease. By enhancing the body’s immune response against the tumor, ICIs offer an additional line of defense and extended survival beyond the initial effects of chemotherapy.
For patients with relapsed or refractory SCLC, treatment options remain limited, and responses to subsequent lines of therapy are often less durable than in the frontline setting. The standard salvage treatment for relapsed SCLC has been topotecan, which offers modest benefits, with overall survival outcomes remaining suboptimal. Lurbinectedin was approved in this setting in 2020.
Tarlatamab: A Breakthrough for Relapsed and Metastatic SCLC
One of the most promising recent developments in the treatment of SCLC is the approval of tarlatamab, a bispecific T-cell engager (BiTE) targeting delta-like ligand 3 (DLL3). This immunotherapy represents a novel approach to treating relapsed or metastatic SCLC.
Tarlatamab engages the immune system more directly in the fight against SCLC by binding to two targets simultaneously: DLL3, which is highly expressed on the surface of SCLC cells, and CD3, a receptor on T cells. By bringing these two cell types into close proximity, tarlatamab enables the T cells to attack and destroy the cancer cells. This mechanism is particularly promising for SCLC, as DLL3 is expressed in approximately 85% of real-world SCLC cases.
DeLLphi-301 Clinical Trial
The Food and Drug Administration based its May 2024 approval of tarlatamab based on the results of the DeLLphi-301 phase II clinical trial. The trial enrolled 220 patients who had previously received at least two prior therapies, including chemotherapy and immune checkpoint inhibitors. Researchers tested two doses, 10 mg and 100 mg of tarlatamab administered intravenously every two weeks.
Results from the trial were hopeful, particularly for the lower-dose (10 mg) group, where a 40% overall response rate (ORR) was observed—far surpassing the historical response rate of 15% seen with standard treatments in this setting. Further, responses were durable. At 10.6 months of follow-up, 57.5% of responders experienced six months or more of response. Subsequent analysis found 30% of responders sustained disease control for at least nine months. The median overall survival for patients in the 10 mg group reached 14.3 months, a significant improvement over the six to 12 months seen with previous treatments. Median duration of response was not reached.
In the DeLLphi-301 trial, the most common side effects were cytokine release syndrome (CRS) and neurologic toxicities, which are characteristic of T-cell engager therapies. CRS was generally mild and manageable, with only 0.8% of patients experiencing grade 3 events on the 10 mg dose and 5.7% on the 100 mg dose. There were no grade 4 or 5 events. Neurologic toxicities, including headache and dizziness, were similarly manageable with appropriate monitoring and dose adjustments.
The 10 mg dose demonstrated similar efficacy to the higher dose with fewer adverse events. As a result, the 10 mg dose has been selected for further study in ongoing clinical trials.
“After decades of minimal advancements in the SCLC treatment landscape, there is now an effective and innovative treatment option available,” Laurie Fenton Ambrose, co-founder, president, and CEO of GO2 for Lung Cancer, said in an Amgen press release. “Today’s FDA approval marks a significant milestone for the SCLC community as the availability of a targeted bispecific therapy brings forward new possibilities to those living with this aggressive disease.”
GO2 for Lung Cancer is the result of a 2019 merger between the two of the most effective and influential nonprofit organizations serving the lung cancer community, the Bonnie J. Addario Lung Cancer Foundation (ALCF) and Lung Cancer Alliance (LCA). It provides a wide range of support for cancer patients, including research updates.
- Peters S, Pujol J-L, Dafni U, et al: Consolidation nivolumab and ipilimumab versus observation in limited-disease small-cell lung cancer after chemo-radiotherapy—Results from the randomised phase II ETOP/IFCT 4-12 STIMULI trial. Ann Oncol 33:67-79, 2022
- Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Kazarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Shire N, Jiang H, Goldman JW; CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019 Nov 23;394(10212):1929-1939. doi: 10.1016/S0140-6736(19)32222-6. Epub 2019 Oct 4. PMID: 31590988.
- Azar I, Austin A, Saha BK, Kim S, Jang H, Sbihi AA, Alkassis S, Yazpandanah O, Chi J, Dhillon V, Mehta HJ, Chopra A, Neu K, Mehdi SA, Mamdani H. The Role of Surgery in Stage I Small Cell Lung Cancer: A National VA Database Analysis. Clin Lung Cancer. 2023 Jul;24(5):e179-e186. doi: 10.1016/j.cllc.2023.04.002. Epub 2023 Apr 15. PMID: 37217388.
- Paz-Ares L, Ahn M, Felip E, et al. Tarlatamab for patients (pts) with previously treated small cell lung cancer (SCLC): Primary analysis of the phase II DeLLphi-301 study. Presented at: European Society for Medical Oncology Congress 2023; October 20-24, 2023; Madrid. Abstract LBA92.
- Sands J, Cho BC, Ahn MJ, et al. Tarlatamab sustained clinical benefit and safety in previously treated SCLC: DeLLphi-301 phase 2 extended follow-up. Presented at: 2024 World Conference on Lung Cancer; September 7-10, 2024; San Diego. Abstract OA10.03.
- Ahn MJ, Cho BC, Felip E, Korantzis I, et. Al.; DeLLphi-301 Investigators. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N Engl J Med. 2023 Nov 30;389(22):2063-2075. doi: 10.1056/NEJMoa2307980. Epub 2023 Oct 20. PMID: 37861218.


