Timothy Hoffman, PhD, a radiopharmaceutical chemist, prepares the tracer drug being used to show whether prostate cancer has spread in the veterans participating in a clinical trial at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO. VA photo by Mindy Roettgen
SAN FRANCISCO — Since receiving Food and Drug Administration (FDA) approval in 2022 for use in metastatic castration-resistant prostate cancer (mCRPC), adoption of lutetium Lu 177 vipivotide tetraxetan (Lu177-PSMA) has grown rapidly. To guide its use outside of clinical trials, the Society of Nuclear Medicine & Molecular Imaging released a consensus statement last year on patient selection and appropriate use of Lu177-PSMA.
As a novel type of treatment, PSMA radioligand therapy raised a number of questions for clinicians, who may be uncertain which patients are eligible and would most benefit from it. Radioligand therapy offers a fourth path for mCRPC patients beyond androgen-focused therapies, chemotherapies and therapies that target specific mutations.
Lu177-PSMA is indicated for use by the FDA for patients with mCRPC who have received chemotherapy with a taxane, either docetaxel or cabazitaxel, and one or more lines of androgen-receptor pathway inhibitors (ARPIs), typically enzalutamide or abiraterone, which are approved for use after chemotherapy.
Beyond that, the consensus statement aimed to guide clinicians in the selection of patients for Lu177-PSMA who are not “perfect,” that is, like most patients seen at the VA or any other real-world clinic, they would not meet the eligibility criteria for the pivotal study on which the drug’s approval was based, or subsequent studies conducted to expand its indications.1
As chief of Nuclear Medicine at the San Francisco VAMC, the lead author of the statement, Thomas A. Hope, MD, knows the range of comorbidities and challenges facing veterans with mCRPC. As a leading researcher on Lu177-PSMA, Hope also has real-world experience with the radioligand in a broad population. In addition to his work at the VA, Hope is a professor of radiology and vice chair of Clinical Operations and Strategy in the Department of Radiology at the University of California-San Francisco (UCSF) School of Medicine.
The panel started with the eligibility for the VISION trial, which was the basis for Lu177-PSMA’s FDA approval. It required all participants to demonstrate prostate-specific membrane antigen (PSMA) positivity, as shown by greater uptake of Gallium-68-PSMA than in the liver for all measurable lesions on PSMA PET imaging. The panel noted that another FDA approved imaging agent, piflufolastat F-18, appeared to work as well for PSMA PET imaging and patient selection and could also be used.
Further, it recommended imaging patients with either contrast-enhanced CT or MRI to identify any PSMA-negative disease, particularly in patients with liver disease. Lu177-PSMA may be an appropriate choice for patients with both PSMA-positive and negative lesions, if the disease is primarily PSMA-positive. “This is particularly true in patients who have completed all available therapeutic options,” noted Hope and colleagues.
Overall, the panel took the approach that most patients who have mCRPC and have received chemotherapy and ARPI for it are going to be quite sick. For many of them, Lu177-PSMA is the last available option, and arbitrary values should not preclude treatment.
In the older men among whom mCRPC is most common, for instance, kidney dysfunction is a very common issue. The panel consensus established a minimum baseline glomerular filtration rate of 30 mL/min. Below that, a multidisciplinary tumor board should weigh in and, if the decision to proceed is made, the marrow should be closely monitored because of the disproportionate impact on marrow in the setting of reduced kidney function. The panel did not recommend presumptive dose reduction based on kidney function, although it may be appropriate in some cases.
Likewise, many men who have metastatic disease and have received multiple lines of cytotoxic therapy will have significantly impaired bone marrow function. Tumor may have replaced marrow in some instances.
Given these realities, the baseline requirements for marrow function set by the consensus included a hemoglobin level of at least 8 g/dL, a white blood cell count of at least 2.0 x 109/L, or an absolute neutrophil count of at least 1.0 × 109/L and a platelet count of at least 75 × 109/L.
The panel cautioned that, with rapidly progressing marrow disease, treatment initiation should not be delayed pending recovery of marrow function, which, in that setting, may not occur.
The panel also agreed that diffuse marrow disease does not make a patient ineligible for PSMA radioligand therapy (RLT). These patients should be monitored closely during treatment, and clinicians should be prepared to provide transfusions as needed.
Real-World Experience with Lu177-PSMA
Hope and his colleagues at UCSF also published real-world results for the use of Lu177-PSMA. The initial report included 99 patients, of whom 60 achieved a 50% or greater reduction in PSA (PSA50). Notably, 19 of the 31 patients who did not meet the laboratory criteria for the VISION trial reached PSA50, as did seven of 18 patients who did not meet the imaging criteria in the TheraP trial (which precluded any PSMA-negative disease).2
Nineteen patients delayed or stopped treatment with Lu177-PSMA based on their strong response. Ten of those patients restarted treatment, and nine again achieved PSA50.
A further 14 participants delayed or discontinued therapy as a result of disease progression. The most common adverse events were anemia, experienced by one-third of the patients, and thrombocytopenia, which affected 21% of those treated.
Based on these results, Hope and his team concluded that patients who did not meet the eligibility criteria for the two largest trials of Lu177-PSMA, either based on imaging or laboratory results, still benefited from treatment with the radioligand.
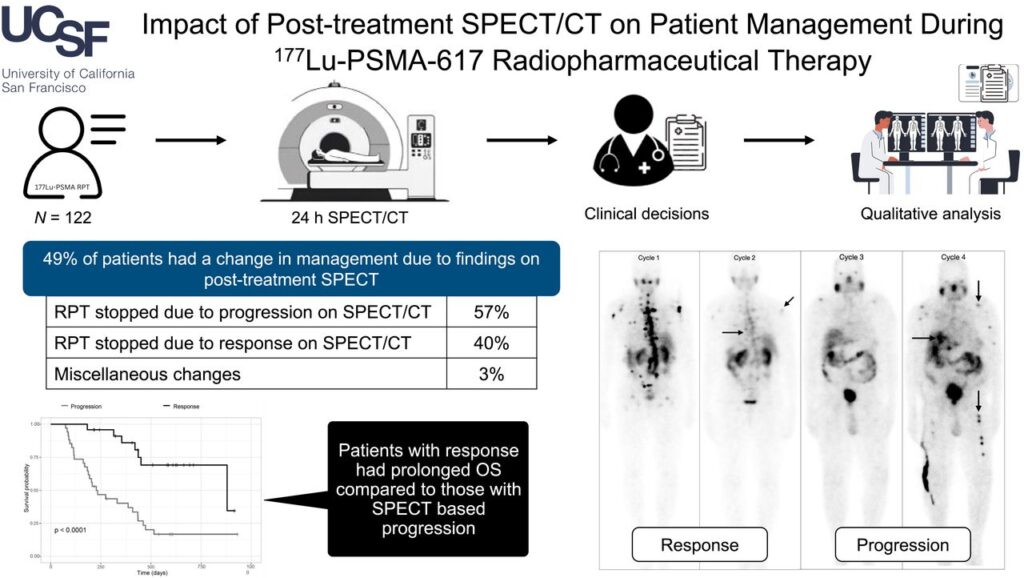
In August, Hope and the UCSF team provided an additional update based on their experience using post-treatment SPECT for management of patients receiving Lu177-PSMA.3
“Nearly 50% of patients had a change in treatment! Post-treatment imaging has a huge impact, and allows us to follow disease at each treatment,” Hope said in a LinkedIn post. “Post-treatment imaging is a unique aspect of lutetium-based theranostics, which is markedly cheaper than PET-based imaging response.”
Of the 122 patients who received the radioligand, 28% stopped treatment because of disease progression. In another 20%, the team delayed or spread out treatment due to good response.
Overall, 42% to 56% exhibited stable disease, while 19% to 39% showed response based on visual assessment across treatment cycles. Sixty patients had changes in their disease management driven by SPECT imaging, with 40% of the changes due to positive response. Changes occurred most often following cycles 2 and 4 of a six-cycle regimen.
- Hope TA, Antonarakis ES, Bodei L, Calais J, Iravani A, Jacene H, Koo PJ, Morgans AK, Osborne JR, Tagawa ST, Taplin ME, Sartor O, Morris MJ. SNMMI Consensus Statement on Patient Selection and Appropriate Use of 177Lu-PSMA-617 Radionuclide Therapy. J Nucl Med. 2023 Sep;64(9):1417-1423. doi: 10.2967/jnumed.123.265952. Epub 2023 Jun 8. PMID: 37290800.
- Moradi Tuchayi A, Yadav S, Jiang F, Kim ST, Saelee RK, Morley A, Juarez R, Lawhn-Heath C, Wang Y, de Kouchkovsky I, Hope TA. Real-World Experience with 177Lu-PSMA-617 Radioligand Therapy After Food and Drug Administration Approval. J Nucl Med. 2024 May 1;65(5):735-739. doi: 10.2967/jnumed.123.266842. PMID: 38485274.
- Yadav S, Lowery B, Tuchayi AM, Jiang F, Saelee R, Aggarwal RR, Juarez R, Flavell RR, Hope TA. Impact of Posttreatment SPECT/CT on Patient Management During 177Lu-PSMA-617 Radiopharmaceutical Therapy. J Nucl Med. 2024 Aug 8:jnumed.124.267955. doi: 10.2967/jnumed.124.267955. Epub ahead of print. PMID: 39117452.