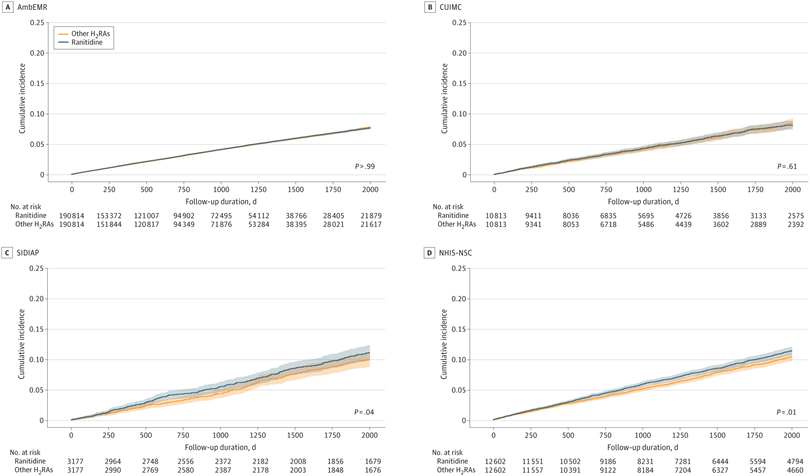
Click to Enlarge: P-values in survival curves were estimated using Cox proportional hazard regression models. Shading in the survival curves indicates 95% CIs. AmbEMR indicates IQVIA US Ambulatory Electronic Medical Research; CUIMC, Columbia University Irving Medical Center data warehouse; NHIS-NSC, Korean National Health Insurance System-National Sample Cohort; SIDIAP, The Information System for Research in Primary Care. Source: JAMA Network Open
SEOUL — In 2020, the U.S. Food and Drug Administration asked manufacturers to remove from the market all products containing ranitidine, a histamine-2 receptor antagonist (H2RA) that has been widely used to treat gastroesophageal reflux disease and peptic ulcer disease.
The action was especially significant because, in the United States, more than 14 million ranitidine prescriptions were issued annually from 2013 to 2018. In 2018, ranitidine was the third most prescribed gastrointestinal medication. The FDA said that some ranitidine medications contained N-nitrosodimethylamine (NDMA), a known human carcinogen, and that those NDMA concentrations increased over time when the products were stored at temperatures higher than room temperature.
Despite the fear that consumers had been exposed to unacceptable levels of the carcinogen, a large new study involving VA researchers has suggested that a history of ranitidine use is not associated with an increased risk of cancer compared with other H2 receptor antagonists.
VA Informatics and Computing Infrastructure in Salt Lake City participated in the study with several Korean and U.S. universities, including UCLA, Columbia and Stanford, as well as other international researchers. The study team has called for further research on the long-term effects of ranitidine on cancer development.
The cohort study included nearly 1.2 million people from 11 large databases across Europe, North America and Asia. Despite past concerns, ranitidine use was not associated with an increased risk of esophageal, stomach, or colorectal cancer, or 13 other subtypes of cancer. The results were published in JAMA Network Open.1
“Ranitidine, the most widely used histamine-2 receptor antagonist (H2RA), was withdrawn because of N-nitrosodimethylamine impurity in 2020. Given the worldwide exposure to this drug, the potential risk of cancer development associated with the intake of known carcinogens is an important epidemiological concern,” the authors wrote.
In response, the study team sought to examine the comparative risk of cancer associated with the use of ranitidine vs. other H2RAs. The new-user active comparator international network cohort study was conducted using 3 health claims and 9 electronic health record databases from the United States, the United Kingdom, Germany, Spain, France, South Korea and Taiwan. Participants were at least 20 years old with no history of cancer and used H2RAs for more than 30 days from January 1986 to December 2020, with a 1-year washout period. Data were analyzed from April to September 2021.
The main exposure was the use of ranitidine as compared to other H2RAs including famotidine, lafutidine, nizatidine and roxatidine
The primary outcome was the development of any cancer types, excluding nonmelanoma skin cancer. Secondary outcomes included the development of any cancer types (including nonmelanoma skin cancer and excluding thyroid cancer) or the 16 cancer subtypes analyzed separately—breast cancer; prostate cancer; lung cancer; colorectal cancer; bladder cancer; liver cancer; leukemia; pancreatic cancer; stomach cancer; lip, oral cavity and pharynx cancer; thyroid cancer; corpus uteri cancer; ovary cancer; esophageal cancer; gall bladder and biliary tract cancer; and cervix uteri cancer.
Of the study participants, 909,168 individuals were identified as new users of ranitidine, and 274, 831 individuals were identified as new users of other H2RAs. The groups were similar, with mean ages in the late 50s and slightly more than half women.
Cancer Rates
Results indicate that crude incidence rates of cancer were 14.30 events per 1,000 person-years (PYs) in ranitidine users and 15.03 events per 1000 PYs among other H2RA users. After PS matching, researchers determined that cancer risk was similar in ranitidine compared with other H2RA users (incidence, 15.92 events per 1000 PYs vs 15.65 events per 1000 PYs; calibrated meta-analytic hazard ratio, 1.04; 95% CI, 0.97-1.12). No significant associations were found between ranitidine use and any secondary outcomes after calibration.
“This cohort study found no consistent association of ranitidine use with cancer risk compared with use of other H2RAs,” the researchers reported. “To our knowledge, this is the first international multi database study and the largest and most comprehensive analysis of the association between ranitidine use and cancer risk, including more than 900 000 users of ranitidine and more than 270 000 users of active comparators from the US, Europe, and Asia.”
The study team cautioned, however, that, while ranitidine use was not associated with increased cancer risk in the meta-analysis, the results from South Korean and Spanish administrative data sources or the meta-analysis from Asia demonstrated an increase in cancer risk of less than <20% in the ranitidine group compared with the other H2RAs group. “This inconsistency in the results prevented us from ruling out a potential association between ranitidine use and cancer development, particularly in certain ethnic groups or health care systems,” the study pointed out.
The researchers posited that the “observed lack of significant association between ranitidine use and cancer risk may be due to low levels of NDMA in ranitidine products. Although a large amount of NDMA would be harmful, NDMA levels in ranitidine found in preliminary tests conducted by the FDA barely exceeded the amount found in common foods.”
“Given the worldwide popularity of ranitidine, exposure of several populations to NDMA and the potential risk of cancer development are important epidemiological concerns, and cancer screening may be required in individuals with previous prolonged exposure to ranitidine,” the authors concluded. “However, the risk of cancer among individuals who used NDMA-contaminated ranitidine has not been fully evaluated. Although some studies have attempted to address this issue, the results are probably underpowered and lack generalizability due to using a single data source. Hence, a large-scale, multinational, multicenter cohort study was conducted to determine whether ranitidine use was associated with increased cancer risk.”
- You SC, Seo SI, Falconer T, et al. Ranitidine Use and Incident Cancer in a Multinational Cohort. JAMA Netw Open. 2023;6(9):e2333495. doi:10.1001/jamanetworkopen.2023.33495


