Inhaled Glucocorticoids Promising in Previous Studies
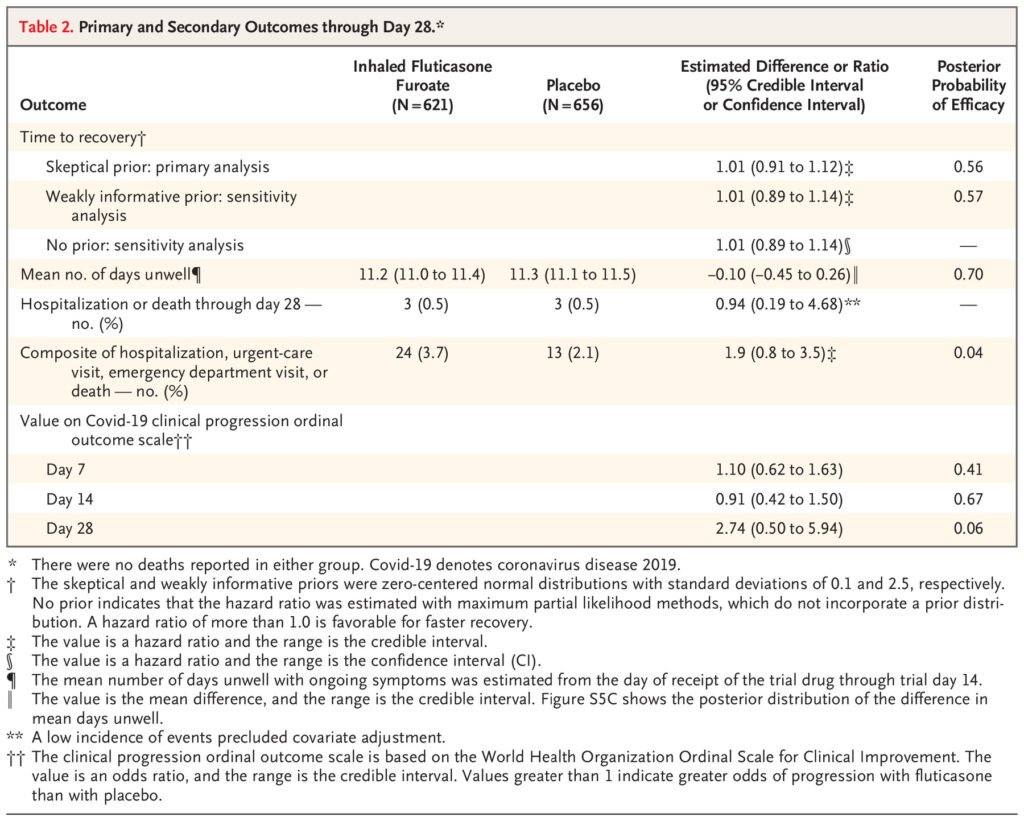
Click to Enlarge: Primary and Secondary Outcomes through Day 28.* Source: The New England Journal of Medicine
MINNEAPOLIS — Inhaled glucocorticoids do not appear to be effective in shortening the time to symptom resolution or preventing hospitalization or death among outpatients with mild-to-moderate COVID-19, according to a recent study.
The New England Journal of Medicine report discusses the results of a decentralized, double-blind, randomized, placebo-controlled platform trial conducted in the United States. The study was designed to assess the use of repurposed medications in outpatients with confirmed infection with SARS-CoV-2.1
University of Minnesota researchers and colleges randomly assigned nonhospitalized adults 30 years of age or older who had at least two symptoms of acute infection that had been present for no more than 7 days before enrollment to receive inhaled fluticasone furoate at a dose of 200 μg once daily for 14 days or placebo.
The research was part of the ACTIV-6 study, where Susanna Naggie, MD, of Duke University School of Medicine, Durham, NC, is the principal investigator.
Defined as the primary outcome was the time to sustained recovery, Hospitalization or death by day 28 and a composite outcome of the need for an urgent-care or emergency department visit or hospitalization or death through day 28 were listed as key secondary outcomes.
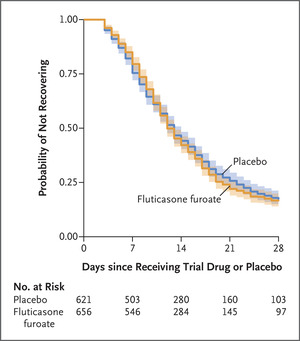
Click to Enlarge: Times to Sustained Recovery with Inhaled Fluticasone Furoate or Placebo. Source: The New England Journal of Medicine
The authors advised that, of the 1,407 enrolled participants who underwent randomization, 715 were assigned to receive inhaled fluticasone furoate and 692 to receive placebo, and 656 and 621, respectively, and were included in the analysis.
“There was no evidence that the use of fluticasone furoate resulted in a shorter time to recovery than placebo (hazard ratio, 1.01; 95% credible interval, 0.91 to 1.12; posterior probability of benefit [defined as a hazard ratio >1], 0.56),” according to the researchers. “A total of 24 participants (3.7%) in the fluticasone furoate group had urgent-care or emergency department visits or were hospitalized, as compared with 13 participants (2.1%) in the placebo group (hazard ratio, 1.9; 95% credible interval, 0.8 to 3.5). Three participants in each group were hospitalized, and no deaths occurred. Adverse events were uncommon in both groups.”
The study team reported that treatment with inhaled fluticasone furoate for 14 days did not result in a shorter time to recovery than placebo among U.S. outpatients with COVID-19.
“As the coronavirus disease 2019 (COVID-19) pandemic continues, the need for early therapies to prevent progression to severe disease is ongoing,” the authors suggested. “New oral antiviral therapies are being used to an increasing degree in high-income countries, with a resulting benefit in unvaccinated persons. However, antivirals are unavailable in most low- and middle-income countries, and vaccination rates are variable. Thus, effective therapies are still needed for persons with symptomatic infection to hasten clinical recovery.”
They note that two open-label randomized trials “involving the use of inhaled budesonide at a dose of 800 μg twice daily for 14 days showed benefits for faster time to recovery and strong trends in decreased hospitalizations or deaths. Conversely, three randomized trials of inhaled ciclesonide (at a dose of 640 μg per day), two of which were double-blind trials, showed no change in symptom duration, and the analyses showed variable decreases—or possible increases—in the use of health care resources.”
The researchers further pointed out that the five trials of inhaled glucocorticoids were conducted among predominantly unvaccinated persons. Because of conflicting results, regulatory authorities and guideline committees did not recommend inhaled glucocorticoid therapy for use as an early treatment option in COVID-19.
The current study team sought to investigate inhaled fluticasone furoate in a double-blind, randomized, placebo-controlled platform trial to assess the use of repurposed drugs in nonhospitalized patients with milder symptoms. “Fluticasone propionate has approximately four times the relative systemic steroid potency of budesonide, and fluticasone furoate has greater receptor affinity than the propionate ester, allowing for once-daily dosing,” they wrote.
”Although no deaths occurred and hospitalizations were rare and similar in both groups, the number of urgent-care and emergency department visits was higher in the fluticasone furoate group than in the placebo group,” the researchers noted. “Overall, the lack of treatment effect and the possible increase in health care visits observed with inhaled fluticasone furoate as compared with placebo (3.2% vs. 1.6%) suggest that inhaled fluticasone furoate is not a favorable COVID-19 therapy.”
They raised the possibility that, because fluticasone and budesonide are different glucocorticoids, “here could be a budesonide-specific effect.” The researchers also pointed out that vaccinated participants in the previous inhaled glucocorticoids trials fared better.
“Our trial did not identify a clinically relevant effect associated with inhaled fluticasone furoate at a daily dose of 200 μg for 14 days as outpatient treatment for COVID-19 when delivered directly to the participant along with written instructions for use of the inhaler,” the study concluded. “We also did not observe a faster time to clinical recovery with fluticasone furoate as compared with placebo in the population studied, unlike results shown in previous open-label trials of inhaled steroids, nor did we observe an effect on prevention of clinical progression.”
- Boulware DR, Lindsell CJ, Stewart TG, Hernandez AF, et. al. Naggie S; ACTIV-6 Study Group and Investigators. Inhaled Fluticasone Furoate for Outpatient Treatment of Covid-19. N Engl J Med. 2023 Sep 21;389(12):1085-1095. doi: 10.1056/NEJMoa2209421. PMID: 37733308.

