VA Study: Hematologic Cancers, Immunological Therapies Lower Effectiveness
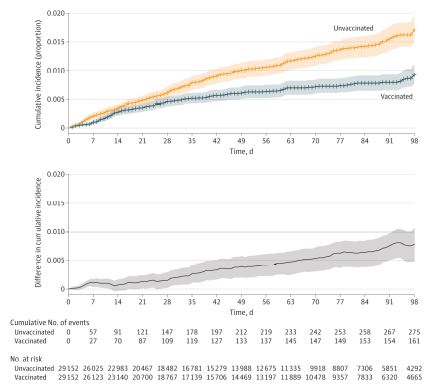
Click to Enlarge: Cumulative Incidence of SARS-CoV-2 Infection After First Vaccine Dose in the Overall Matched CohortCumulative incidence curves of SARS-CoV-2 infection in the overall matched cohort using time zero as the date of the first dose of vaccination. The difference in cumulative incidence is illustrated in the gray bottom graph. Shaded areas indicate 95% CIs calculated by bootstrapping.
BOSTON — With new variants popping up and steady occurrence of breakthrough infections, the question of the effectiveness of SARS-CoV-2 vaccination in patients with cancer has not really been answered until now.
“Although patients with cancer have poor COVID-19–related outcomes, the effectiveness of SARS-CoV-2 vaccination in this population is unclear, because they were excluded from vaccination trials,” according to new research. “Recent studies suggest that patients with cancer mount less robust antibody responses to vaccination than immunocompetent controls. The objective of this retrospective cohort study in the national Veterans Affairs (VA) health care system was to estimate the SARS-CoV-2 vaccination effectiveness in patients with cancer in a real-world setting during the 140-day period following initial vaccine availability.”
For the cohort of VA patients who received systemic therapy for cancer between Aug.15, 2010, and May 4, 2021, a proxy measure for effectiveness of the vaccine starting 14 days after the second dose was determined to be 58%. In addition, VA authors and colleagues pointed out that the measure of effectiveness beginning 14 days after the second dose was 85% in patients who had not received systemic therapy within the six months prior to vaccination and 76% among those who had received hormonal treatment.
The study team included researchers from the VA Palo Alto, CA, Healthcare System; Stanford University School of Medicine in Stanford, CA; the VA Boston Healthcare System, as well as Harvard Medical School and Dana-Farber Cancer Institute, both in Boston.
“Results suggest that SARS-CoV-2 vaccination associated with lower infection rates in patients with cancer, especially in those not receiving current systemic therapy and those receiving hormonal treatment,” the authors wrote in JAMA Oncology.1
The article discussed how patients with cancer are at increased risk for severe COVID-19, but it has remained unknown whether SARS-CoV-2 vaccination actually is effective for them.
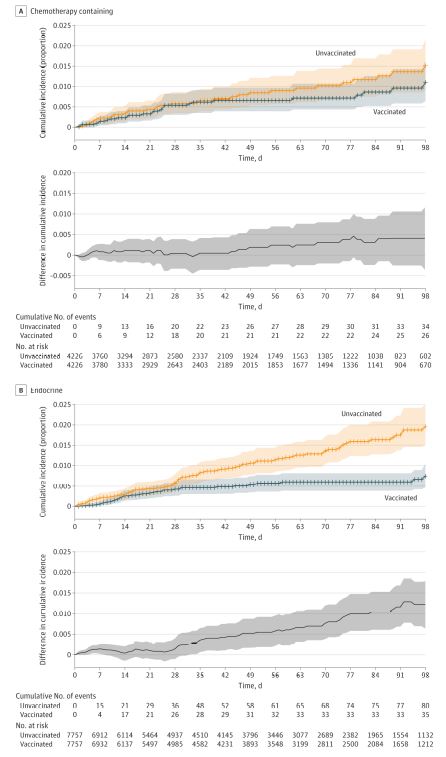
Click to Enlarge: Cumulative Incidence of SARS-CoV-2 Infection After First Vaccine Dose in Patients Receiving Current Treatment With Chemotherapy-Containing Regimens and Endocrine Therapy Cumulative incidence curves of SARS-CoV-2 infection among patients receiving current treatment with (A) a chemotherapy-containing regimen and (B) an endocrine therapy regimen at the time of vaccination. Time zero is the date of the first dose of vaccination or entry as an unvaccinated control. The difference in cumulative incidence is illustrated in the gray bottom graphs. Shaded areas indicate 95% CIs calculated by bootstrapping. Current treatment is defined as systemic therapy received within the 3 months prior to vaccination (if in the vaccinated group) or prior to entry date (if in the unvaccinated group).
To help answer the question, the study team sought to determine the association between SARS-CoV-2 vaccination and SARS-CoV-2 infections among a population of VA patients with cancer.
Their retrospective, multicenter, nationwide cohort study of SARS-CoV-2 vaccination and infection among VHA patients was conducted from Dec. 15, 2020, to May 4, 2021. Included were all adults with solid tumors or hematologic cancer who received systemic cancer-directed therapy from Aug. 15, 2010, to May 4, 2021, and were alive and without a documented COVID-19 positive result as of Dec. 15, 2020.
For the study, researchers each day matched newly vaccinated patients 1:1 with unvaccinated or not yet vaccinated controls based on age, race and ethnicity, VA facility, rurality of home address, cancer type and treatment type/timing.
Defined as the primary outcome was documented SARS-CoV-2 infection. A proxy for vaccine effectiveness was defined as 1 minus the risk ratio of SARS-CoV-2 infection for vaccinated patients compared with unvaccinated controls.
Of the 184,485 patients who met eligibility criteria, 113, 796 were vaccinated. Of these, 29,152 vaccinated patients—median [IQR] age, 74.1 [70.2-79.3] years; 95% were men; 71% were non-Hispanic white individuals—were matched 1:1 to unvaccinated or not yet vaccinated controls.
Lower Death Rate
Researchers reported that, as of a median 47 days of follow-up, 436 SARS-CoV-2 infections were detected in the matched cohort—161 infections in vaccinated patients vs. 275 in unvaccinated patients. While 17 COVID-19-related deaths occurred in the vaccinated group, 27 were documented in the unvaccinated group.
“Overall vaccine effectiveness in the matched cohort was 58% (95% CI, 39% to 72%) starting 14 days after the second dose,” the authors wrote. “Patients who received chemotherapy within 3 months prior to the first vaccination dose were estimated to have a vaccine effectiveness of 57% (95% CI, -23% to 90%) starting 14 days after the second dose vs. 76% (95% CI, 50% to 91%) for those receiving endocrine therapy and 85% (95% CI, 29% to 100%) for those who had not received systemic therapy for at least six months prior.”
While, in general, COVID-19 vaccination was associated with lower SARS-CoV-2 infection rates in patients with cancer, researchers cautioned, “Some immunosuppressed subgroups may remain at early risk for COVID-19 despite vaccination, and consideration should be given to additional risk reduction strategies, such as serologic testing for vaccine response and a third vaccine dose to optimize outcomes.”
The authors also said their study was the first to deal with the issue of vaccine effectiveness against SARS-CoV-2 infection in patients with cancer, adding, “Vaccine effectiveness may decrease with age, and malignancy and anticancer treatments may be immunosuppressive. These factors may account for the reduced effectiveness we observe among some patients with cancer vs. effectiveness reported in the general population recently estimated to be 89% to 92%.”
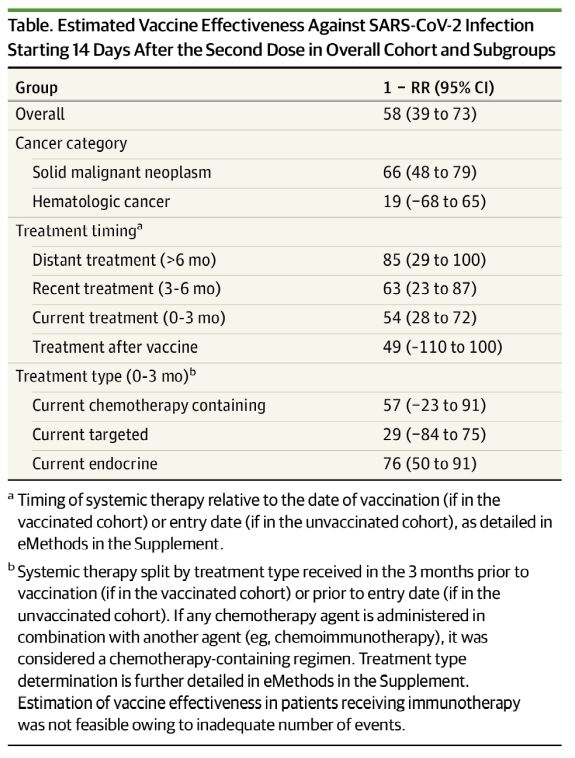
Click to Enlarge: Estimated Vaccine Effectiveness Against SARS-CoV-2 Infection Starting 14 Days After the Second Dose in Overall Cohort and Subgroupsa Timing of systemic therapy relative to the date of vaccination (if in the vaccinated cohort) or entry date (if in the unvaccinated cohort), as detailed in eMethods in the Supplement.
The study helped clear up some confusions, finding, for example, that vaccine effectiveness in patients receiving endocrine therapy and those last receiving therapy more than six months before vaccination was similar to general population estimates.
The results also pinpointed some problem areas. “We independently identify previously demonstrated risk factors for poor vaccination response,” researchers noted. “hematologic cancers and immunosuppressive therapies are associated with reduced antibody response to vaccination and lower effectiveness in our study. Our findings suggest that high-risk populations may benefit from additional mitigation measures, such as extended masking. Data from other immunosuppressed populations suggest that a third vaccine dose may improve effectiveness. Additional work is needed to determine efficacy of a similar approach in patients with cancer, particularly those who were vaccinated during active treatment.”
The study’s most significant limitation, according to the authors, is that, because of limited power, they could not fully assess vaccination effectiveness against severe COVID-19 and COVID-19-related deaths. “However, we found a reduction in SARS-CoV-2 infections, suggesting that vaccines mitigate against these important outcomes,” they said.
The study team called for additional studies” including a different patient population with longer follow-up period will be valuable, as will studies to delineate optimal vaccination regimen and administration relative to cancer and treatment type.”
- Wu JT, La J, Branch-Elliman W, et al. Association of COVID-19 Vaccination With SARS-CoV-2 Infection in Patients With Cancer: A US Nationwide Veterans Affairs Study. JAMA Oncol. Published online December 02, 2021. doi:10.1001/jamaoncol.2021.5771

