LONG BEACH, CA — Hepatitis C can lead to serious liver diseases including cirrhosis and liver cancer—particularly hepatocellular carcinoma, which accounts for about 90% of all liver cancer cases. Infection with either hepatitis B or hepatitis C are significant risk factors for developing hepatocellular carcinoma.1
In 2014, the VHA, in recognition of an increased prevalence of hepatitis C among its patients, launched the Hepatic Innovation Team (HIT) Collaborative. The goal of this program was to implement systemic changes that would increase the number of patients being tested for infection and treated with recently-approved direct-acting antiviral drugs.
“Previously, the available treatments had a lot of side effects and were not very effective. The cure rate was low, and very few veterans were treated,” says Timothy Morgan, MD, of the VA Long Beach Healthcare System and director of the VA National Liver Disease Program. “Because of the HIT Collaborative as well as support from Congress and VA Central Office, the VA was very effective at supporting all of its facilities in testing veterans and getting them treated with these very effective new drugs.”
The program was so successful—treating more than 85% of veterans known to be infected with hepatitis C—that, in 2018, the HIT Collaborative expanded to focus on cirrhosis care and early detection of hepatocellular carcinoma as well as treatment for hepatitis C. (Although hepatocellular carcinoma surveillance hasn’t conclusively been shown to reduce liver cancer death, the American Association for the Study of Liver Diseases recommends screening adults with cirrhosis every six months.) A new study published in Cancers details the achievements of this ongoing national program, which has engaged teams of providers across the U.S. to improve patient care. It was led by researchers from the Center for Health Equity Research and Promotion at the VA Pittsburgh Healthcare System.2
“Even after they were cured of the infection, many veterans with hepatitis C were left with cirrhosis of the liver,” explained Morgan, the study’s senior author. “In considering how to best manage patients who had received hepatitis treatment and other veterans with cirrhosis, one of the HIT Collaborative’s main aims was to increase surveillance for hepatocellular carcinoma.”
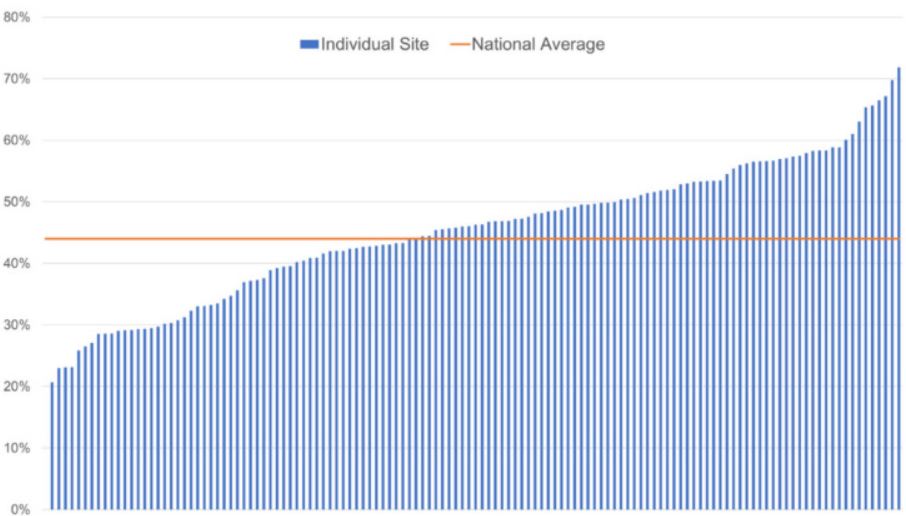
Click to Enlarge: Proportion of Veterans receiving hepatocellular carcinoma surveillance in fiscal year 2018, by facility.
The HIT Collaborative promoted two data tools: the Advanced Liver Disease (ALD) Dashboard and the Hepatocellular Carcinoma (HCC) Clinical Reminder. The ALD Dashboard is a population management tool that providers can use to identify veterans with liver disease in their facilities and assess whether they have been recently screened for hepatocellular carcinoma. The HCC Clinical Reminder pops up in a veteran’s electronic medical record when it’s time for the patient to be screened.
In addition, the HIT Collaborative leadership team regularly engaged 18 regional, multidisciplinary teams that included hepatologists, pharmacists, infectious disease providers, systems redesign experts, implementation scientists and health services researchers. They hosted monthly calls to share lessons learned and provide quarterly data. Between meetings and calls, regional teams could access the presentations and receive technical assistance and coaching from the leadership team. The leadership team provided education about the ALD Dashboard and HCC Clinical Reminder, trained clinicians in systems redesign methods, and developed a tool to estimate the cost of facility-level cirrhosis care. And the leadership team offered on-site visits, when they worked with regional teams to set goals, assess data, and develop quality improvement strategies.
System Barriers
“We knew there were system barriers,” Morgan told U.S. Medicine. “The goal was to try to help facilities identify some of those barriers and try to overcome them.”
By September 2018, the ALD Dashboard had been used by 96% of facilities across all 18 regions. Between 2018 and 2019, the first two years of the HIT’s program focused on cirrhosis, adoption of the HCC Clinical Reminder doubled from 16% to 30%. Surveillance rates for hepatocellular carcinoma also significantly increased, from 46% to 51%, over the course of those two years.
The study’s found the HIT collaborative was key to this success. The facilities that showed the most improvement were those that utilized the program’s tools, including the ALD Dashboard and the HCC Clinical Reminder, and had at least one member in the HIT Collaborative. Even smaller facilities without on-site gastroenterology or hepatology specialists were able to increase their surveillance rates using the HIT tools and supports.
“We are still working toward a higher surveillance rate,” Morgan advised. “But when you compare VA to non-VA facilities, we’re doing pretty darn well.” The study noted that, in other healthcare systems, the percentage of patients receiving consistent surveillance has been reported to be as low as 2% to 11%, and a recent meta-analysis puts the rate at 24%.
“Why is the rate so low? That is a complicated question that we continue to try to answer,” Morgan suggested. “It’s hard to change things. It takes constant effort and there is not a one-size-fits-all solution for every medical center. You need to have local connections and local input and local support to be able to address their issues. That is what the HIT Collaborative is designed to do—to work with local VA facilities to identify and overcome barriers to care.”
- Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021 May;18(5):293-313. doi: 10.1038/s41575-020-00395-0. Epub 2021 Jan 28. PMID: 33510460.
- Rogal SS, Yakovchenko V, Gonzalez R, Park A, Beste LA, Rozenberg-Ben-Dror K, Bajaj JS, Scott D, McCurdy H, Comstock E, Sidorovic M, Gibson S, Lamorte C, Nobbe A, Chartier M, Ross D, Dominitz JA, Morgan TR. The Hepatic Innovation Team Collaborative: A Successful Population-Based Approach to Hepatocellular Carcinoma Surveillance. Cancers (Basel). 2021 May 7;13(9):2251. doi: 10.3390/cancers13092251. PMID: 34067177; PMCID: PMC8125814.