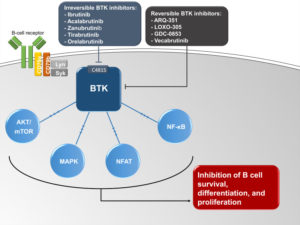
A simplified schematic of the role of Bruton’s tyrosine kinase (BTK) in B cell receptor signaling and B cell survival. Ibrutinib, acalabrutinib, zanubrutinib, tirabrutinib, and orelabrutinib are irreversible BTK inhibitors that inactivate BTK by binding to C481S. ARQ-351, LOXO-305, GDC-0853, and vecabrutinib are reversible BTK inhibitors that inactivate BTK independent of C481S.
Abbreviations: AKT/mTOR, mammalian target of rapamycin; LYN, Lck/Yes kinase; MAPK, mitogen-activated protein kinase; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; SYK, spleen tyrosine kinase.
COLUMBUS, OH – Mantle cell lymphoma (MCL), an uncommon B-cell non-Hodgkin lymphoma, has an aggressive clinical course in the majority of patients and remains uncurable. It also is extremely challenging to treat, according to a new review.
Over the last few years, Bruton’s tyrosine kinase (BTK) inhibitors have become the preferred treatment option for patients with relapsed/refractory MCL, according to a recent journal article. Also good candidates for the therapy are patients considered unfit for chemotherapy or those with chemo-resistant disease.
The Ohio State University Comprehensive Cancer Center-led researchers pointed out that, for most patients, treatment is required at the time of diagnosis, with age, performance status, comorbidities, and patient/physician’s preference all playing a role in therapy decisions. Writing in OncoTargets & Therapy, the authors advised, “Younger fit patients are typically treated with intensive chemotherapy (generally defined as regimens including high-dose cytarabine) with or without consolidative autologous hematopoietic cell transplantation (HCT), whereas older or unfit patients are treated with less-intensive chemotherapy. Maintenance with rituximab is commonly considered in both approaches.”1
“Both intensive and less-intensive approaches result in high response rates that exceed 80% to 90%, but intensive chemotherapy results in deeper responses and longer remissions,” they added. “However, even in patients treated with intensive chemotherapy, relapses are inevitable with 4- to 6-year progression-free survival (PFS) of 50% to 65%.”
With relapsed MCL a major therapeutic challenge, six non-chemotherapy agents are currently approved in the United States and/or Europe for the treatment of patients with relapsed/refractory MCL: bortezomib, temsirolimus, lenalidomide, and three Bruton’s tyrosine kinase (BTK) inhibitors: ibrutinib, acalabrutinib, and zanubrutinib.
“Of these agents, the BTK inhibitors are generally considered the preferred treatment option for patients with relapsed/refractory MCL as they have the highest response rates and are generally well-tolerated,” according to the review, which added that three BTK inhibitors are currently approved by the Food and Drug Administration (FDA) in relapsed/refractory MCL: ibrutinib, acalabrutinib, and zanubrutinib.
The authors emphasized out that the second-generation BTK inhibitors, acalabrutinib and zanubrutinib, “were designed to have less off-target inhibition than ibrutinib and an enhanced safety profile. Beyond safety, the improved selectivity of the second-generation BTK inhibitors might impact their efficacy and potential for combination therapy.”
The authors called for more research comparing first- and second-generation BTK inhibitors. “Zanubrutinib is now the third irreversible BTK inhibitor approved for patients with relapsed/refractory MCL. Cross-trial comparisons show similar efficacy for ibrutinib, acalabrutinib, and zanubrutinib in relapsed/refractory MCL,” they wrote. “However, the toxicity profiles of these BTK inhibitors appear to vary, and the more selective BTK inhibitors zanubrutinib and acalabrutinib may be associated with a lower incidence of some toxicities of interest including cardiovascular toxicity. Results of ongoing randomized clinical trials comparing acalabrutinib and zanubrutinib with ibrutinib will be important.”
- Sawalha Y, Bond DA, Alinari L. Evaluating the Therapeutic Potential of Zanubrutinib in the Treatment of Relapsed/Refractory Mantle Cell Lymphoma: Evidence to Date. Onco Targets Ther. 2020 Jul 6;13:6573-6581. doi: 10.2147/OTT.S238832. Erratum in: Onco Targets Ther. 2020 Sep 04;13:8009. PMID: 32753893; PMCID: PMC7351990.

