VHA Issued Controversial Internal Memo on Issue Last Year
 WASHINGTON – The Food and Drug Administration has approved an updated label for the Pfizer-BioNTech vaccine, urging that the sixth dose in vaccine vials be used.
WASHINGTON – The Food and Drug Administration has approved an updated label for the Pfizer-BioNTech vaccine, urging that the sixth dose in vaccine vials be used.
That information settles once and for all a controversy that arose late last year after a VHA internal memorandum was made public and drew strong criticism.
The question now is whether enough of the special syringes required to access the sixth doses will be available.
Despite that, Pfizer has said the sixth dose would be considered in meeting its commitment to the United States of 200 million Covid-19 vaccine doses, which now would be achieved by the end of May, two months sooner than previously expected.
Connecting Vets, a broadcast and media production company, obtained an internal VHA memo issued in December that initially directed pharmacy chiefs and others not to use the sixth dose.
In the memo, VHA said it was waiting for FDA guidance but dictated that, until then, sites should only use five doses per vial, and that any discarded doses didn’t have to be tracked as waste in the pharmacy benefits management tracker.
The memo suggested that “drawing extra doses will make the data questionable, as there is no way to truly confirm which vials have extra fill and which don’t,” adding, “There is a risk that some sites may try to combine overfill from multiple vials. These vials are preservative-free and the practice may pose an infection risk.”
The memo published by Connecting Vets also stated that, because of uncertainty about which vials have six doses, allocations could be affected that would leave some veterans unable to get a second dose.
After the Connecting Vets article was disseminated – pointing out that the VHA’s initial guidance was issued despite the FDA tentatively approving use of all full doses available in each vial during the public health emergency — and several members of Congress criticized the agency policy, it was reversed..
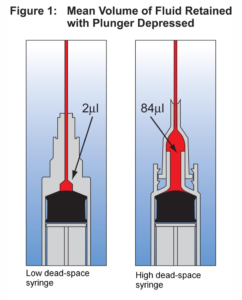
At the end of January, the FDA approved an updated label stating, “Low dead-volume syringes and/or needles can be used to extract six doses from a single vial. If standard syringes and needles are used, there may not be sufficient volume to extract a sixth dose from a single vial.”
The FDA also changed the following: “After dilution, one vial contains 6 doses of 0.3 mL. Vial labels and cartons may state that after dilution, a vial contains 5 doses of 0.3 mL. The information in this Fact Sheet regarding the number of doses per vial after dilution supersedes the number of doses stated on vial labels and cartons.”
Regulators further stated that each dose must contain 0.3 mL of vaccine, explaining that, if the amount of vaccine remaining in the vial cannot provide a full dose of 0.3 mL, the vial and content should be discarded, and excess vaccine should not be pooled from multiple vials.
Although federal officials say that some low dead-volume syringes will be shipped with Pfizer-BioNTech vaccine allotments, many vaccinators might find they need extra. That is likely to be a problem, at least in the short-term.
Manufacturers have said that producing more of the special needles was not a priority for the Trump Administration, so they directed their efforts to other products. Now, to fill supply shortfalls, President Joe Biden has said he will invoke the Defense Production Act (DPA). A White House press release said Biden issued an executive order which “directs agencies to fill supply shortfalls using all available legal authorities, including the DPA, and the United States has identified twelve immediate supply shortfalls that will be critical to the pandemic response, including shortages in the dead-space needle syringes available to administer the vaccine.”
Other areas that might have production increases through the DPA include N95 masks, isolation gowns, nitrile gloves, polymerase chain reaction (PCR) sample collection swabs, test reagents, pipette tips, laboratory analysis machines for PCR tests, high-absorbency foam swabs, nitrocellulose material for rapid antigen tests and rapid test kits.


