COLUMBIA, SC—A VA study has put to rest, at least temporarily, claims that hydroxychloroquine, alone or in combination with azithromycin, is highly effective in treating COVID-19 infection.
In fact, a study appearing on the medRxiv preprint server identified increased overall mortality in veterans hospitalized for novel coronavirus infection and treated with hydroxychloroquine alone.1
Research was conducted by the Dorn Research Institute at the Columbia, SC, VA Health Care System, the University of South Carolina and the University of Virginia.
“Despite limited and conflicting data on the use of hydroxychloroquine in patients with COVID-19, the U.S. Food and Drug Administration has authorized the emergency use of this drug when clinical trials are unavailable or infeasible,” the authors wrote, adding that anecdotal information suggests that hydroxychloroquine, alone or in combination with azithromycin, is being widely used in COVID-19 therapy.
Researchers sought to determine if the therapy affected mortality rates or the need for mechanical ventilation.
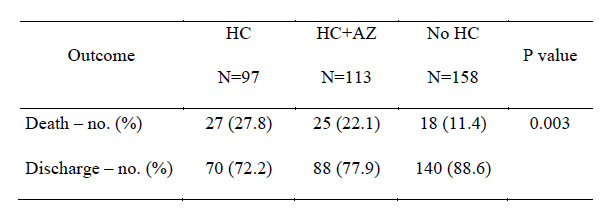
Outcomes based on treatment exposure.
HC: hydroxychloroquine-treated
HC+AZ: hydroxychloroquine and azithromycin-treated
No HC: not treated with hydroxychloroquine
To do that, the study team performed a retrospective analysis of data from 368 patients hospitalized with confirmed SARS-CoV-2 infection in all VHA medical centers until April 11, 2020. The review divided patients in groups based on their exposure to hydroxychloroquine alone or with azithromycin as treatments in addition to standard supportive management for COVID-19. Of the participants, 97 were in the HC group, 113 in the HC+AZ group, and the remaining 158 only received standard supportive care.
Results indicated that rates of death in the HC, HC+AZ and no HC groups were 27.8%, 22.1%, 11.4%, respectively, while rates of ventilation in the HC, HC+AZ and no HC groups were 13.3%, 6.9% and 14.1%, respectively.
“Compared to the no HC group, the risk of death from any cause was higher in the HC group (adjusted hazard ratio, 2.61; 95% CI, 1.10 to 6.17; P=0.03) but not in the HC+AZ group (adjusted hazard ratio, 1.14; 95% CI, 0.56 to 2.32; P=0.72),” the authors reported. “The risk of ventilation was similar in the HC group (adjusted hazard ratio, 1.43; 95% CI, 0.53 to 3.79; P=0.48) and in the HC+AZ group (adjusted hazard ratio, 0.43; 95% CI, 0.16 to 1.12; P=0.09), compared to the no HC group.”
Researchers said they found “no evidence that use of hydroxychloroquine, either with or without azithromycin, reduced the risk of mechanical ventilation in patients hospitalized with COVID-19. An association of increased overall mortality was identified in patients treated with hydroxychloroquine alone. These findings highlight the importance of awaiting the results of ongoing prospective, randomized, controlled studies before widespread adoption of these drugs.”
Hydroxychloroquine, an antimalarial used to treat some rheumatoid arthritis and lupus patients, has been promoted as potentially beneficial in patients with COVID-19, including by President Donald Trump, although some medical experts have expressed skepticism.
The FDA’s Emergency Use Authorization was issued for hydroxychloroquine sulfate supplied from the Strategic National Stockpile to treat adults and adolescents who weigh 50 kg or more and are hospitalized with COVID-19 for whom a clinical trial is not available or participation is not feasible. The agency said the suggested dose to treat adults and adolescents who weigh 50 kg is 800 milligrams of hydroxychloroquine sulfate on the first day of treatment and then 400 milligrams daily for four to seven days of total treatment based on clinical evaluation.
Trump announced the stockpile of 29 million doses of hydroxychloroquine for use in COVID-19 patients.
The findings of the veteran study generally were in line with other unpublished research released at about the same time. An earlier, multicenter, open-label, randomized controlled trial in China sought to assess the efficacy and safety of hydroxychloroquine plus standard-of-care compared with SOC alone in adult patients with COVID-19.
That study was conducted in government-designated COVID-19 treatment centers in China from Feb. 11 to Feb. 29, 2020, in 150 patients hospitalized with COVID-19, according to a report in medRxiv.2
For half of the patients, HCQ was administrated with a loading dose of 1,200 mg daily for three days followed by a maintained dose of 800 mg daily for the remaining days, with the total treatment duration of two or three weeks for mild/moderate or severe patients, respectively. The remaining patients only received standard of care.
Researchers were focused on the 28-day negative conversion rate of SARS-CoV-2, as well as negative conversion rate at Days 4, 7, 10, 14 or 21, the improvement rate of clinical symptoms within 28-day, normalization of C-reactive protein and blood lymphocyte count within 28-day.
Results suggested that the overall 28-day negative conversion rate was not different between the SOC plus HCQ and SOC groups (Kaplan-Meier estimates 85.4% vs. 81.3%, P=0.341). Negative conversion rate at Days 4, 7, 10, 14 or 21 was also similar between the two groups, and no difference in 28-day symptoms alleviation rate was observed between the two groups.
On the other hand, a significant efficacy of HCQ on alleviating symptoms was observed when the confounding effects of anti-viral agents were removed in the post-hoc analysis (Hazard ratio, 8.83, 95%CI, 1.09 to 71.3). “This was further supported by a significantly greater reduction of CRP (6.986 in SOC plus HCQ vs. 2.723 in SOC, milligram/liter, P=0.045) conferred by the addition of HCQ, which also led to more rapid recovery of lymphopenia, albeit no statistical significance,” according to the authors.
Adverse events were found in 8.8% of SOC and 30% of HCQ recipients with two serious adverse events; diarrhea was the most common adverse event in the HCQ recipients.
“The administration of HCQ did not result in a higher negative conversion rate but more alleviation of clinical symptoms than SOC alone in patients hospitalized with COVID-19 without receiving antiviral treatment, possibly through anti-inflammatory effects,” researchers wrote. “Adverse events were significantly increased in HCQ recipients but no apparent increase of serious adverse events.”
In the United States, cardiologists had cautioned about the potential for increased cardiovascular issue with hydroxychloroquine and azithromycin. A report in the American Heart Association’s journal Circulation pointed out that both drugs are considered definite causes of torsade de pointes. “There are occasional case reports of hydroxychloroquine prolonging the QT interval and provoking torsade de pointes when used to treat systemic lupus erythematosus,” the authors wrote, adding that azithromycin, a widely used antibiotic, is increasingly recognized as a rare cause of QT prolongation, serious arrhythmias, and increased risk for sudden death.
- Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, Ambati J. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv 2020.04.16.20065920; doi: https://doi.org/10.1101/2020.04.16.20065920
- Tang W, Cao Z, Han M, Wang Z, et. Al. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. medRxiv 2020.04.10.20060558; doi: https://doi.org/10.1101/2020.04.10.20060558
- Roden DM, Harrington RA, Poppas A. Russo AM. Considerations for Drug Interactions on QTc in Exploratory COVID-19 (Coronavirus Disease 2019) Treatment. Originally published Originally published8 Apr 2020 https://doi.org/10.1161/CIRCULATIONAHA.120.047521.


