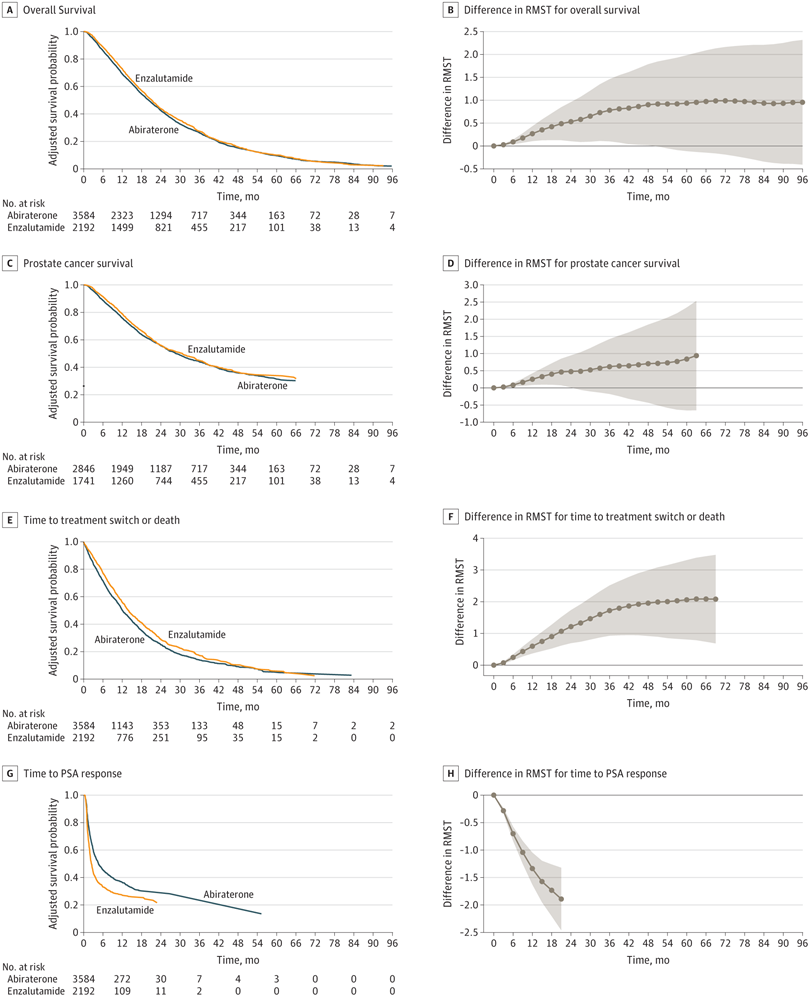
Click to Enlarge: Kaplan-Meier plots and the difference in restricted mean survival time (RMST) at each time point (3-month increments) are shown. The RMST at a given time point measures the mean survival censoring at the time point and is equal to the area under the Kaplan-Meier plot up to the time point. PSA indicates prostate-specific antigen. Source: JAMA Network Open
WASHINGTON — As the options for treatment of metastatic castration-resistant prostate cancer (mCRPC) continues to increase, determining the best order for sequencing them becomes more complicated. Fortunately, the VA’s oncology clinical pathways for prostate cancer, the National Comprehensive Cancer Network guidelines and recent research provide assistance.
A recent VA study raises the possibility of guidance changes for the selection of first- and second-line therapies, but, perhaps the biggest news, is the expansion of alternatives in the second- and even third-line. Patients who do not have targetable mutations or progress on androgen receptor signaling inhibitors now have radioligands as an option. Further, considering the potential need for a second- or third-line therapy might affect earlier choices.
The VA recommends two radiopharmaceuticals in the second line, lutetium Lu 177 vipivotide tetraxetan (Lu177-PSMA) and radium-223.
Lu177-PSMA is a beta-emitting radiopharmaceutical that binds to prostate-specific membrane antigens (PSMA) on the surface of prostate cancer cells. Beta radiation penetrates deeply but is less damaging to non-targeted tissues. Lu-177-PSMA is Food and Drug Administration (FDA)-approved for use in patients who have previously received androgen receptor pathway inhibition and taxane-based chemotherapy.
Lu177-PSMA received FDA approval for use with Gallium-68 (Ga-68) PSMA, which is used to detect PSMA-positive metastases. Ga-68 PSMA enables visualization of prostate cancer metastases on PET/CT imaging very early in their formation, well before a tumor would ordinarily be seen on imaging.
The FDA based its approval of Lu177-PSMA on the VISION trial. That study randomized 831 patients with PSMA-positive mCRPC confirmed via PSMA PET/CT scans on a 2:1 basis to receive Lu177-PSMA plus standard of care (SOC) or SOC alone. At a median follow-up of 20.9 months, the Lu177-PSMA arm had 60% improved radiographic progression-free survival, 8.7 months compared to 3.4 months for SOC alone (HR: 0.40 [99.2% CI: 0.29–0.57]; p<0.001).1
Median overall survival was 38% better at 15.3 months for the Lu177-PSMA arm vs. 11.3 months for the SOC only arm (HR: 0.62 [95% CI: 0.52–0.74]; p<0.001). More than half of patients receiving Lu177-PSMA who had measurable target lesions demonstrated partial or complete response compared to 3% of those in the SOC alone arm. Lu177-PSMA also nearly doubled the time to first skeletal event or death, with a median of 11.5 months vs. 6.8 months with SOC alone (HR: 0.50 [95% CI: 0.40–0.62]).
Lu177-PSMA is also the preferred third-line therapy in the VA pathway for patients who receive one of the other options in the second line, discussed below.
Radium-223 is an alpha-emitting radiopharmaceutical that has shown effectiveness in patients with symptomatic bone metastases, but no visceral involvement. Because of the risk of myelosuppression, it is recommended for use as a monotherapy or in combination only with an androgen-deprivation therapy. It is administered by IV once a month for six months. Radium-223 can increase the risk of bone fractures when administered with abiraterone and prednisone, so the National Comprehensive Cancer Network (NCCN) guidelines advise adding denosumab or zoledronic acid.
Earlier Therapeutic Decisions
For first-line treatment of mCRPC, the VA recommends considering abiraterone for patients with adenocarcinoma with an ECOG performance status of 2 or less, though it is contraindicated in patients with hepatic dysfunction or significant cardiovascular disease and those who cannot tolerate prednisone. For patients who have a BRCA mutation, olaparib may be added to abiraterone.
For individuals not eligible for abiraterone, the VA pathways recommend docetaxel or enzalutamide. Docetaxel is preferred for patients with relatively rapid doubling times but is associated with greater toxicity. A palliative care referral should be considered for patients with adenocarcinoma with ECOG greater than 2.
A VA study published in JAMA Network Open in August might lead to revision of that recommendation in the next draft of the pathways, however. Researchers at the VA Boston Healthcare System and their colleagues conducted a retrospective, multicenter study of veterans treated with abiraterone or enzalutamide over an eight-year period. They found “small but statistically significant improvements” in overall survival, prostate cancer-specific survival, time to initiation of next treatment or death, and time to prostate-specific antigen response for veterans who received enzalutamide compared to those who received abiraterone.2
In particular, veterans who had PSA doubling time of three months or more and those who did not first receive docetaxel benefited the most. Enzalutamide initiation was associated with 1.14 months (95% CI, 0.19-2.10 months) longer restricted mean survival time (RMST) in overall survival in docetaxel-naïve patients and 2.23 months longer (95% CI, 0.81-3.66 months) in those with longer PSA doubling times. Both prior docetaxel and relatively rapidly progressing disease obviated those gains.
Clinicians might also want to consider their future options in choosing between the androgen receptor pathway inhibitors (ARPI) enzalutamide and abiraterone, however. The PSMAfore trial demonstrated that Lu177-PSMA doubled radiographic progression-free survival (rPFS) compared to a change to a second ARPI, regardless of initial selection, in taxane-naïve patients.3
In a post-hoc analysis of the study presented at the 2024 American Urological Association Annual Meeting, though, the results favored starting with abiraterone. Patients who first received abiraterone, then switched to Lu177-PSMA, had a median rPFS of 12.62 months compared to 5.78 months for those who switched to a second ARPI. Among those who received enzulamide first, those who initiated Lu177-PSMA next had a rPFS of 12.02 months vs. 4.34 months for those who switched to a second ARPI.4
The VA and NCCN advise use of platinum-based chemotherapy combination for first-line treatment of patients with mCRPC with a neuroendocrine component. NCCN recommends using cisplatin/etoposide, carboplatin/etoposide, docetaxel/carboplatin or cabazitaxel/carboplatin, but no combination is preferred over the others.
In the second-line for mCRPC of all types, the VA pathway recommends docetaxel for patients who are eligible. If that’s not an option, physicians and patients have several alternatives. These include radioligands Lu177-PSMA and radium 223; ARPIs enzalutamide and abiraterone/prednisone; and cabazitaxel.
For individuals with targetable mutations, the VA recommends pembrolizumab for patients with microsatellite instability-high or mismatch repair-deficient cancers and the PARP-inhibitor olaparib for those with BRCA2 variants or other mutations in DNA repair genes.
Lu177-PSMA is the preferred third-line therapy in the VA pathway. If a patient is not a candidate for it, any of the second-line therapies that have been tried are alternatives.
- Sartor O, de Bono J, Chi KN, Fizazi K, et. Al; VISION Investigators. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103. doi: 10.1056/NEJMoa2107322. Epub 2021 Jun 23. PMID: 34161051; PMCID: PMC8446332.
- La J, Wang L, Corrigan JK, et al. Abiraterone or Enzalutamide for Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA Netw Open. 2024;7(8):e2428444. doi:10.1001/jamanetworkopen.2024.28444
- Sartor AO, Castellano Guana DE, Herrmann K, et al. LBA13 phase III trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore). Annals of Oncology. 2023;34(suppl2):S1324
- Shore ND, Wei XX, Chi KN, et al. Efficacy of [177Lu]Lu-PSMA-617 versus ARPI change in taxane-naive patients with metastatic castration-resistant prostate cancer by pre-randomization ARPI (PSMAfore). J Urol. 2024;211(5S2). doi:10.1097/01.JU.0001015816.87470.c9.04
