No Increase in Mortality Identified
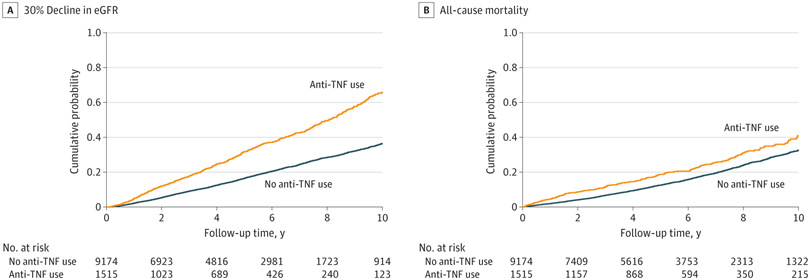
Click to Enlarge: Kaplan-Meier Cumulative Event Curves for at Least 30% Decline in Estimated Glomerular Filtration Rate (eGFR) and All-Cause Mortality Associated With Incident Use (vs Nonuse) of Tumor Necrosis Factor (TNF) Inhibitors in the Overall Cohort.
Log-rank P < .001 for both. Kaplan-Meier curves were demographically adjusted for age, sex, and race.
Source: JAMA Netork Open
MEMPHIS, TN — The introduction of biologic therapies, such as antitumor necrosis factor (TNF) agents, has revolutionized the management of inflammatory bowel disease (IBD)—a group of diseases characterized by chronic inflammation of the gastrointestinal tract. The demonstrated therapeutic benefits of the agents along with efficacy and tolerability have led to their widespread use.
But a study earlier this year by VA researchers suggested a potential downside of anti-TNF agents and points to the importance of monitoring kidney function when initiating anti-TNF therapies in patients with IBD, which includes Crohn’s disease and ulcerative colitis.
Extraintestinal manifestations, such as chronic kidney disease, as well as premature mortality and other adverse clinical outcomes, have been linked to IBD. Although several studies have reported positive effects of anti-TNF therapy on the extraintestinal symptoms of IBD, research on the therapy’s effect on the survival of IBD patients is limited, stated the authors of the new study published in JAMA Network Open. To their knowledge, no prior studies had assessed the drugs’ effect on risk of CKD progression, an established risk factor for mortality.1
To examine the association of incident use of TNF inhibitors with subsequent decline in kidney function and risk of all-cause mortality, the researchers at the Memphis VAMC Center in Tennessee and Tibor Rubin VAMC in Long Beach, CA, conducted a large nationwide cohort study of U.S. veterans with comprehensive patient-level clinical data.
The researchers analyzed longitudinal data from the Therapeutics Interventions to Assess Outcomes and Disparities in CKD study, a nationwide retrospective cohort study of more than 3.5 million US veterans with mildly low to normal estimated glomerular filtration rate (eGFR)—60 mL/min/1.73 m2 or greater—recorded between Oct. 1, 2004, with follow-up through Sept. 30, 2019.
As the researchers employed an incident new-user design to delineate treatment exposure among patients within incident IBD diagnosis, they initially identified 22,179 patients with a new diagnosis of IBD. More than half were excluded from the study however, due to end-stage kidney disease or mortality before the baseline (the date of the first TNF inhibitor prescription or a randomly generated baseline date for patients without TNF inhibitor use) or due to missing data corresponding to the baseline date.
Data were analyzed from December 2022 to February 2024. The main outcomes were at least a 30% decline in estimated glomerular filtration rate (eGFR) and all-cause mortality.
Among the 10,689 patients in the study cohort with incident IBD, the mean age was 67.4, and 93.5% were male; 3,353 (31.4%) had diabetes. The mean baseline eGFR was 77.2 mL/min/1.73 m2, and 1,515 (14.2%) of the cohort were newly initiated on anti-TNF therapy. During a median follow-up of 4.1 years, 3,367 patients experienced at least 30% decline in eGFR, and, over a median follow-up of 5.0 years, 2,502 patients died.
Risk of eGFR Decline
After multivariable adjustments, the researchers found incident use (vs. nonuse) of TNF inhibitors was significantly associated with higher risk of decline in eGFR. “With few exceptions, the association of TNF inhibitor use to the risk of decline in eGFR was more evident in subgroups of patients aged 70 years and older and those without corticosteroid use than in their counterparts,” the authors stated. The use of TNF inhibitors was not associated with risk of all-cause mortality, however.
Although their observational study cannot conclude any causal relationship, the authors said there are at least a few plausible explanations for the observed association between anti-TNF therapy and progressive kidney function decline.
One of these is autoimmune-mediated phenomena—including anti-TNF-induced lupus, a well-recognized clinical entity that affects various organs—which have been documented as common biologically related adverse events in IBD. Another could be related to an increase in serum creatinine generation as a consequence of gaining muscle mass. “Albeit speculative, it is therefore possible that the gain of muscle mass associated with improvement of IBD symptoms following anti-TNF therapy and resultant amelioration of nutritional deficiency led to an apparent decline in eGFR among patients with incident TNF inhibitor use,” they wrote.
While a few observational studies have reported a lower risk of mortality associated with incident TNF inhibitor use, these previous studies have assessed the mortality risk associated with new anti-TNF therapy compared with prolonged use of corticosteroids, the researchers wrote. Thus, it was not clear whether patients with new anti-TNF drug use could have a survival benefit compared with nonusers of such therapy, irrespective of prolonged use of corticosteroids.
In this study, a comparison of mortality risk between those newly prescribed TNF inhibitors and patients who had never used them, regardless of concurrent medications, revealed no notable survival advantage linked with the initiation of TNF inhibitors, indicating a lack of significant correlation between anti-TNF therapy and mortality.
Despite several advantages of the study, the authors acknowledged limitations. Because the cohort was predominantly male veterans, the findings may not be generalizable to female or nonveterans and because of the exposure of interest was TNF inhibitors, the findings might not apply to biologics in other classes. Further, because of the low number of incident kidney failure cases requiring kidney replacement in this cohort, the researchers were unable to examine the association of incident TNF inhibitor use with a tangible kidney outcome.
The researchers concluded that further research is needed to validate their results and examine potentially distinct pathophysiological contributions of incident TNF inhibitor use to kidney and nonkidney outcomes in patients with IBD.
- Sumida K, Shrestha P, Mallisetty Y, Thomas F, et al. Anti-Tumor Necrosis Factor Therapy and Risk of Kidney Function Decline and Mortality in Inflammatory Bowel Disease. JAMA Netw Open. 2024 Apr 1;7(4):e246822. doi: 10.1001/jamanetworkopen.2024.6822. PMID: 38625700; PMCID: PMC11022116.
