Prescriptions for Other AD Therapy Drop After Initiation
Currently, topical ruxolitinib is the only JAK inhibitor therapy approved in North America for the treatment of atopic dermatitis. New specialty group guidelines clarified the optimal use of the product in AD patients unable to get relief from other therapies but also questioned whether it should be used in most patients with mild to moderate disease refractory to moisturization alone. A recent industry study cited other benefits, however.
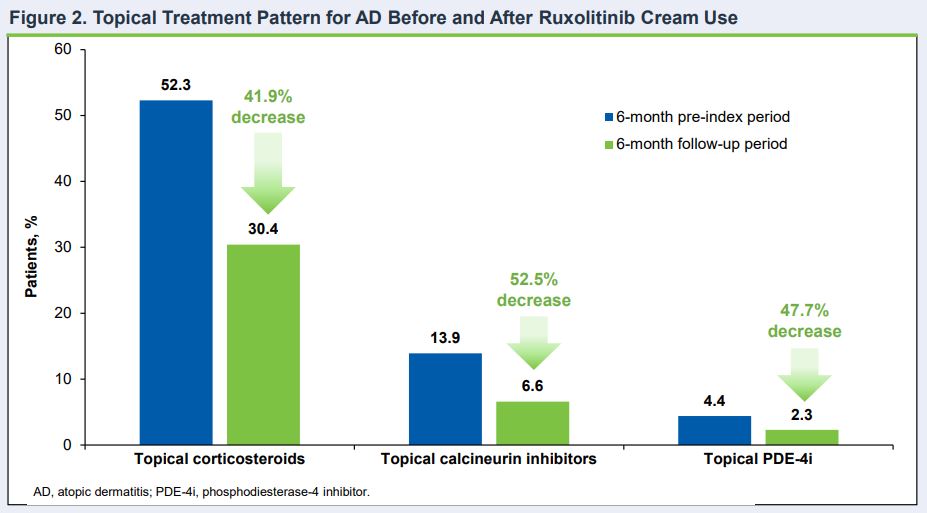
Click to Enlarge: Topical Treatment Pattern for AD Before and After Ruxolitinib Cream Use Source: American Academy of Dermatology (AAD) Annual Meeting
SAN DIEGO — Using topical ruxolitinib cream to treat atopic dermatitis decreases the need for other AD treatments, according to new research.
The new industry study presented at the American Academy of Dermatology annual meeting established that benefit, according to the authors, who noted, “Within the 6 months following ruxolitinib cream initiation, there was a decrease in utilization of other AD treatments.”
Ruxolitinib cream is a topically administered selective Janus kinase (JAK) 1/JAK2 inhibitor approved in the United States in 2021.1
The presentation described it as “an effective nonsteroidal monotherapy initially used twice daily continuously to reduce signs and symptoms of AD, and as-needed for longer-term disease control, as shown in adults and adolescents with mild to moderate AD in two phase 3 clinical studies.”
In the study based on a U.S. payor claims database, almost half of the patients using ruxolitinib cream did not receive any other AD treatment, with both topical and oral corticosteroid use decreasing.
Most, more than 90% of biologics-naive patients did not initiate biologics, and 17.4% of patients who were on biologics during the 6-month pre-index period did not continue these treatments during the 6-month follow-up period, the study pointed out.
“Thus, early assessments suggest that initiating ruxolitinib cream may reduce the overall need for other AD therapies such as topical/oral corticosteroids and biologics,” the study team concluded.
The retrospective observational study was conducted using claims data from the Healthcare Integrated Research Database (HIRD) and included patients with a first claim for ruxolitinib cream between October 2021 and July 2022. The 1,581 participants were 12 or older on the index date, with one or more pharmacy claims of ruxolitinib cream along with a medical claim with an AD diagnosis in the prior 6 months, and six or more months of continuous enrollment in a healthcare plan before and after the index date.
The study’s findings were not considered in recently issued guidelines, however.
Recent recommendations on the management of atopic dermatitis from the American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology clarified recommendations on the use of topical Janus kinase (JAK) inhibitors.
New guidelines said JAK inhibitors such as ruxolitinib might be used in AD patients under certain conditions but should not be in most of those with mild-to-moderate disease refractory to moisturization alone.
“Although many topical JAK inhibitors are in development, only ruxolitinib is currently available in North America,” guideline authors, including a representative from the James A. Haley VA Hospital in Tampa, wrote in the March issue of Annals of Allergy, Asthma & Immunology.2
Their recommendation about the use of the topical JAK inhibitor product in adolescents and adult patients was noted as being conditional, with low-certainty evidence. The guidelines suggested the following conditions be considered:
- “Patients who place a higher value on certain larger benefits and safety profiles of other topical treatments (e.g., TCS 2-4, tacrolimus) and certain systemic therapies are less likely to prefer topical ruxolitinib”.
- “Patients who are immunocompromised, immunosuppressed, or have risk factors for serious infection, cancer, thrombosis, or cardiovascular events may prefer other treatments compared with topical ruxolitinib”.
- “Patients who have not responded to other topical therapies and/or those who highly value the modest benefits of topical ruxolitinib over the more certain larger benefits of other topical treatments, and ruxolitinib’s uncertain association with an increased risk of cancer, thromboembolism, serious infection, and mortality, and safety profile of systemic treatments, might favor topical ruxolitinib”.
The panel pointed out the topical treatments systematic review and NMA, which included three random controlled trials and more than 1,400 adolescent and adult participants with mild AD—defined as mean ∼9.5% body surface area involvement and mean EASI ∼8—comparing, topical ruxolitinib vs. either standard care or TCS 4 (triamcinolone 0.1% cream), revealed high or moderate certainty improvements in AD severity (RD 23 more per 100 [6-41 more]), itch (34 more per 100 [20-47 more]), sleep disturbance (4 more per 100 [0-10 more]) and quality of life (35 more per 100 [25 more to 45 more]).
“Whether topical ruxolitinib reduces flares is highly uncertain due to imprecision and the short-term (4-8 weeks) nature of the available studies that assessed relevant interventions and controls (comparators),” according to the guideline authors, who added, “Topical ruxolitinib is slightly more potent in improving most patient-important AD outcomes compared to pimecrolimus (between TCS 5 and TCS 6/7).”
The article also advised that, overall, adverse events within the time frame were similar between topical ruxolitinib and control groups (RD 5 fewer per 100 [12 fewer to 4 more]). “The direct data were too short and did not contain enough adults (at risk) to credibly estimate the effect on death, cancer, thrombosis, or serious infections,” according to the report. “Stroke was observed in the topical ruxolitinib group in the TRuE-AD trials, but recent data, a mix of observational and randomized data, to 40 weeks suggest favorable safety.”
In 2021, the U.S. Food and Drug Administration announced the approval of ruxolitinib cream, marketed as Opzelura, for the short-term and noncontinuous chronic treatment of mild-to-moderate AD in non-immunocompromised patients aged 12 years and older whose disease is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
Discontinuous Use
Because the FDA placed a boxed warning label on all JAK inhibitors in 2022 due to concerns about increases in major cardiovascular adverse events and cancer, as well as, at higher doses, venous thromboembolism, serious infections and death from any cause, the guideline panel recommended some limitations on use of the topical product. “Concerns about systemic absorption with topical JAK inhibitors are sufficient to limit application of ruxolitinib to less than 20% BSA and use it in a discontinuous manner [to] decrease the potential for harm,” it wrote.
Still, the guidelines recommended the use of oral JAK inhibitors “after careful consideration of risks and possible benefits in adults and adolescents with moderate-severe AD refractory, intolerant, or unable to use mid- to high-potency topical treatment and systemic treatment inclusive of a biologic recommended previously.”
The authors advised, however, that, “in most mild-moderate patients with AD, the risk with a topical JAK inhibitor, however, would be predicted to be lower than that with an oral JAK inhibitor. Robust comparative long-term data are required to definitively clarify serious harms, if any, of using topical ruxolitinib.”
They urged that patients and clinicians considering topical ruxolitinib “should engage in a discussion of the potential benefits and harms and establish whether topical ruxolitinib or another topical or systemic therapy optimally aligns with patient values and preferences.
The panel said it has not yet made an official recommendation for topical calcineurin inhibitors (TCIs) and topical corticosteroids (TCS) vs. ruxolitinib, explaining that “many clinical experts and patients will start with TCS or TCI first. Similarly, clinical experts expressed that although most patients may not prefer ruxolitinib as first-line treatment, it might be a resource to consider for those patients for whom TCS and TCI do not yield sufficient control.”
Guidance, addressing atopic dermatitis (AD) management, was last issued in 2012 by the two specialty groups. The panel agreed on 25 recommendations to gain and maintain control of AD for patients with mild, moderate, and severe AD.
In addition to topical JAK inhibitors, the document advises on optimal use of:
- topical treatments (barrier moisturization devices, corticosteroids, calcineurin inhibitors, PDE4 inhibitors [crisaborole], occlusive [wet wrap] therapy, adjunctive antimicrobials, application frequency, maintenance therapy),
- dilute bleach baths,
- dietary avoidance/elimination,
- allergen immunotherapy,
- systemic treatments (biologics/monoclonal antibodies, small molecule immunosuppressants [cyclosporine, methotrexate, azathioprine, mycophenolate, JAK inhibitors], and systemic corticosteroids) and UV phototherapy (light therapy).
- Liu J, Desai K, Then C-C, Sturm, D. Atopic Dermatitis Treatments Before and After Initiation of Ruxolitinib Cream: Analysis of a US Payer Claims Database. Presented at the American Academy of Dermatology (AAD) Annual Meeting, San Diego, CA, March 8-12, 2024.
- AAAAI/ACAAI JTF Atopic Dermatitis Guideline Panel; Chu DK, Schneider L, Asiniwasis RN, Boguniewicz M, et. Al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE- and Institute of Medicine-based recommendations. Ann Allergy Asthma Immunol. 2024 Mar;132(3):274-312. doi: 10.1016/j.anai.2023.11.009. Epub 2023 Dec 18. PMID: 38108679.
